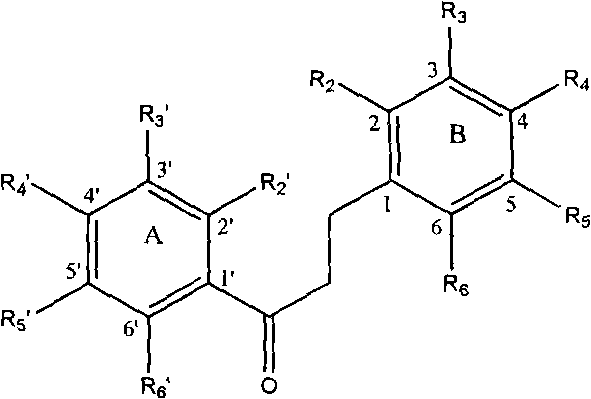
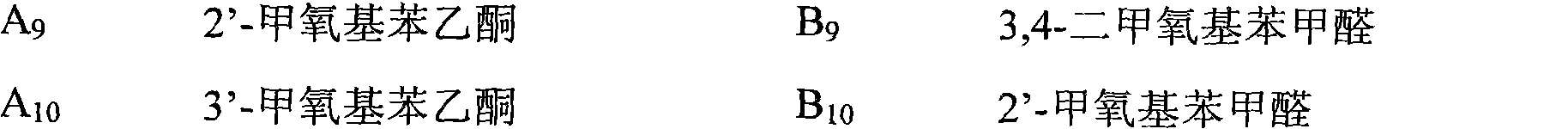Method for screening dihydrochalcone compound
A dihydrochalcone and screening method technology, applied in biological testing, material inspection products, etc., can solve problems such as energy-consuming, time-consuming and cost-consuming, and achieve the effect of reducing time and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
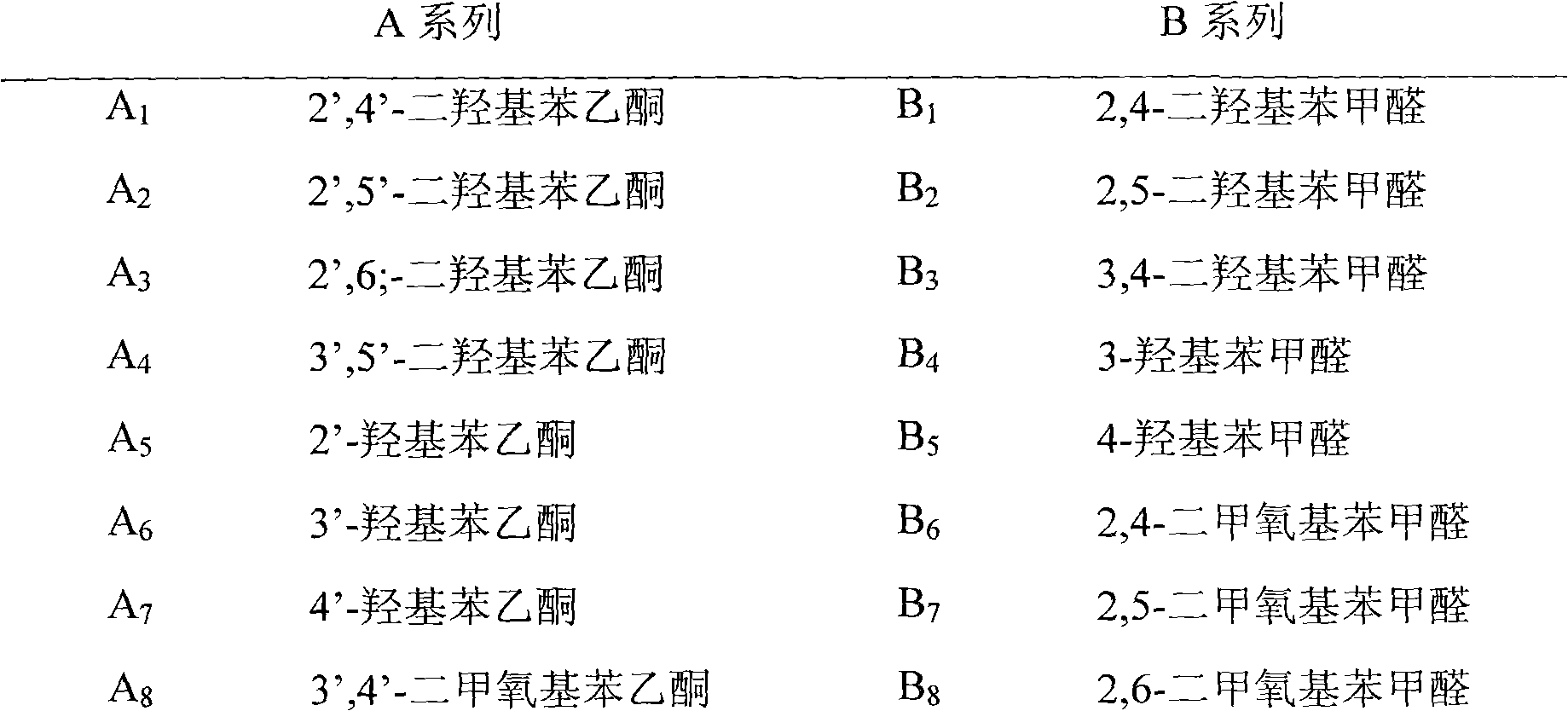
[0025] a. Prepare 10 kinds of acetophenone compound series A and 10 kinds of benzaldehyde compound series B. The names and corresponding serial numbers of the compounds are shown in Table 1 respectively.
[0026] Table 1 A, B series compound names and corresponding serial numbers
[0027]
[0028]
[0029] Before aldol condensation, A 1 to A 7 , B 1 to B 5 The active phenolic hydroxyl group on the benzene ring is protected by a benzyl group, and the steps are as follows: add 0.05mol hydroxyl-containing acetophenone compound or benzaldehyde compound to a 250mL three-necked flask, dissolve it in 50mL absolute ethanol, and then add 0.035mol K 2 CO 3 , under mechanical stirring, slowly add 0.06mol benzyl chloride dropwise, the molar ratio of hydroxyl-containing acetophenone compounds or benzaldehyde compounds to benzyl chloride is 1:1.2, after the addition is completed, heat to reflux for reaction, TLC tracking reaction to Raw material points disappear. Recover ethanol...
Embodiment 2
[0043] a. Prepare 7 kinds of acetophenone compound series A and 12 kinds of benzaldehyde compound series B. See Table 4 for the compound names and corresponding serial numbers.
[0044] Table 4 A, B series compound names and corresponding serial numbers
[0045]
[0046] Before aldol condensation, A 6 and B 11 The active phenolic hydroxyl group on the benzene ring is protected by a benzyl group, and the steps are as follows: add 0.05molA 6 or B 11 , after dissolving with 50mL absolute ethanol, add 0.035mol K 2 CO 3 , under mechanical stirring, slowly drop 0.06mol benzyl chloride, the hydroxyl-containing A 6 or B 11 The molar ratio of benzyl chloride to benzyl chloride is 1:1.2. After the dropwise addition is completed, the reaction is heated to reflux, and the reaction is tracked by TLC until the raw material point disappears. Recover ethanol by rotary evaporation, add water, and extract with ethyl acetate. The extract was washed twice with 5% NaOH, and then washed ...
Embodiment 3
[0061] Take normal Kunming mice, half male and half female, fasted without water for 14 hours, intraperitoneally injected with 2% alloxan (200 mg / kg) normal saline solution, and resume normal diet after injection. After 72 hours, the blood glucose of mice fasted for 8 hours without food and water was measured, and those whose blood glucose value was greater than 11.1mmol / L were experimentally qualified animal models of diabetes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com