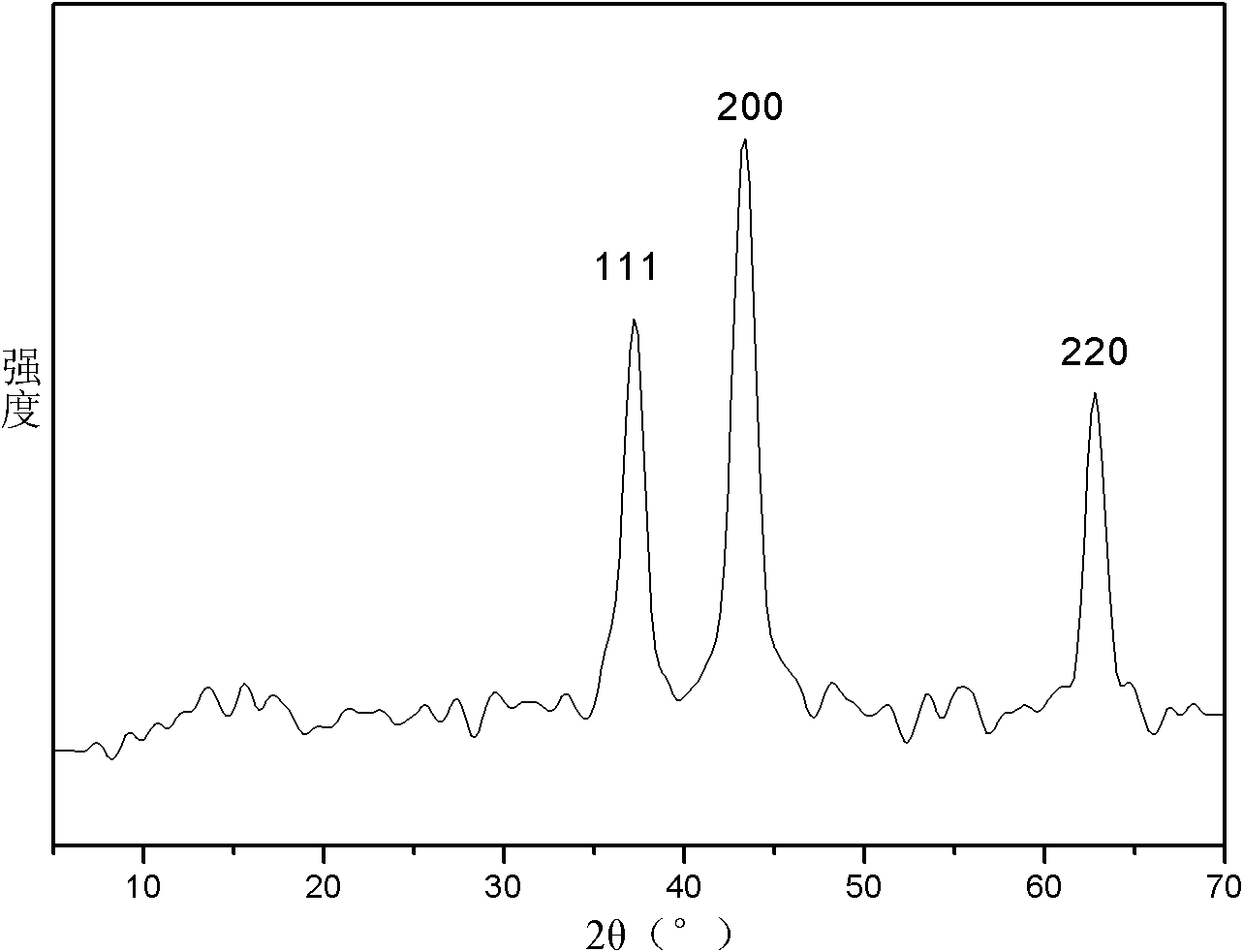
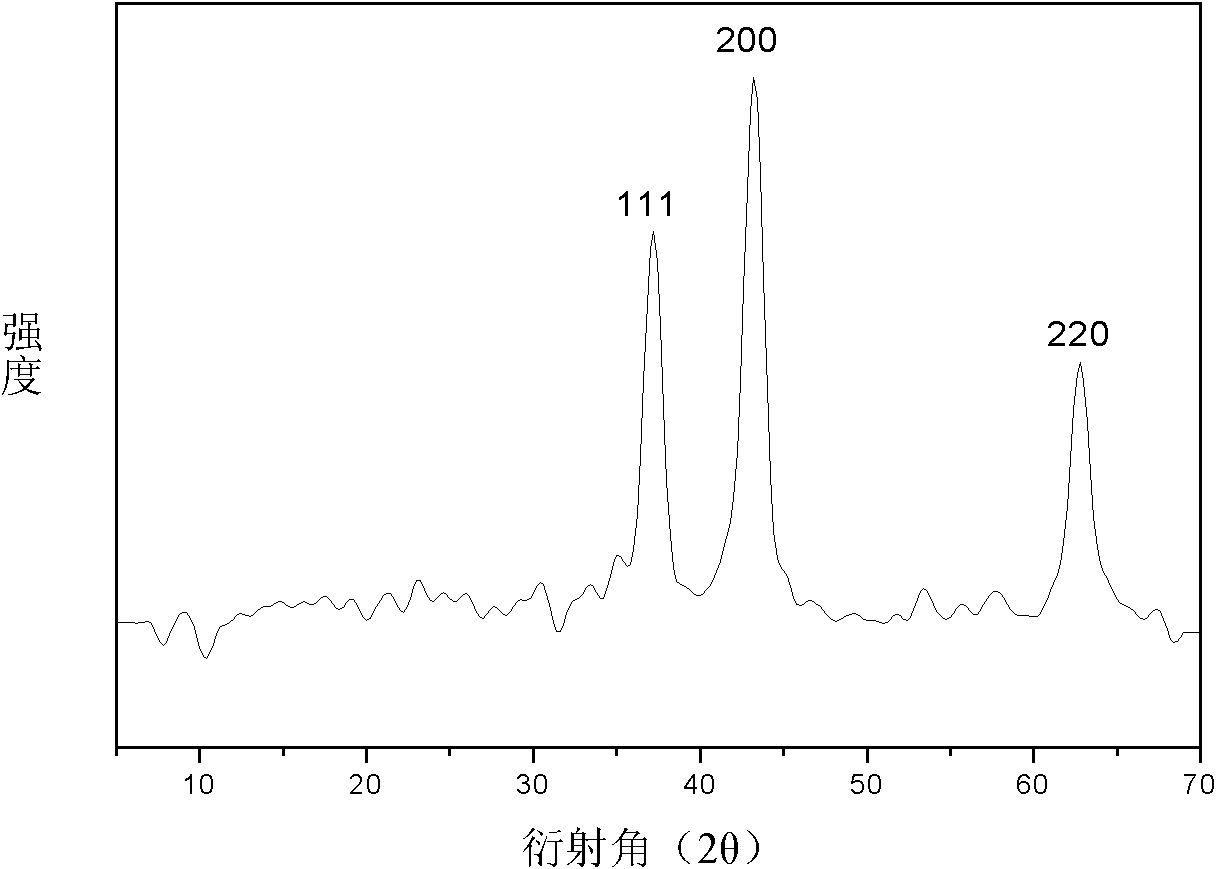
Method for preparing NiO nano flowerlike microspheres with surface topography controllable
A technology of surface morphology and nanoflowers, applied in the direction of nanotechnology, nickel oxide/nickel hydroxide, etc., to achieve stable performance, low price, and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Put 30mL ethylene glycol into a 50mL beaker, add 0.6mL polyethylene glycol (PEG-400), and stir magnetically for 2h; add 10mmol nickel nitrate hexahydrate and 1mmol benzoic acid, stir magnetically for 1h; drop Add 0.6 mL of NaOH solution with a concentration of 2 mol / L, and stir magnetically for 0.5 h to form a blue-green transparent mixed solution.
[0033] (2) The above mixed solution was placed in a 50ml polytetrafluoroethylene-lined stainless steel reaction kettle, sealed, and heated in an oven for 8 hours; the reaction temperature was 160°C.
[0034] (3) Take out the polytetrafluoroethylene pressure vessel and cool it down to normal temperature. Take out the reaction solution, put it into a 50ml centrifuge tube, separate the precipitation product in the reaction solution by centrifugation, and wash it repeatedly with absolute ethanol and deionized water for 3 times, and place the obtained precipitation product in an oven at 60°C Dry to obtain nickel hydroxide p...
Embodiment 2
[0037] (1) Put 30mL ethylene glycol into a 50mL beaker, add 1.2mL polyethylene glycol (PEG-400), and stir magnetically for 2h; add 6mmol nickel nitrate hexahydrate and 3mmol benzoic acid, stir magnetically for 1h; drop Add 0.9 mL of NaOH solution with a concentration of 2 mol / L, and stir magnetically for 0.5 h to form a blue-green transparent mixed solution.
[0038] (2) The above mixed solution was placed in a 50ml polytetrafluoroethylene-lined stainless steel reaction kettle, sealed, and heated in an oven for 10 hours; the reaction temperature was 180°C.
[0039] (3) Take out the polytetrafluoroethylene pressure vessel and cool it down to normal temperature. Take out the reaction solution, put it into a 50ml centrifuge tube, separate the precipitation product in the reaction solution by centrifugation, and wash it repeatedly with absolute ethanol and deionized water for 3 times, and place the obtained precipitation product in an oven at 80°C Dry to obtain nickel hydroxide p...
Embodiment 3
[0042] (1) Put 30mL ethylene glycol into a 50mL beaker, add 1.8mL polyethylene glycol (PEG-400), and stir magnetically for 2h; add 1mmol nickel nitrate hexahydrate and 5mmol benzoic acid, stir magnetically for 1h; drop Add 1.5 mL of NaOH solution with a concentration of 2 mol / L and stir magnetically for 0.5 h to form a blue-green transparent mixed solution.
[0043] (2) The above mixed solution was placed in a 50ml polytetrafluoroethylene-lined stainless steel reaction kettle, sealed, and heated and reacted in an oven for 14 hours; the reaction temperature was 220°C.
[0044] (3) Take out the polytetrafluoroethylene pressure vessel and cool it down to normal temperature. Take out the reaction solution, put it into a 50ml centrifuge tube, separate the precipitated product in the reaction solution by centrifugal separation, and wash it repeatedly with absolute ethanol and deionized water for 3 times, and place the obtained precipitated product in an oven at 100°C Dry to obtain ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com