Caliper type chromium complex and preparation method and application thereof
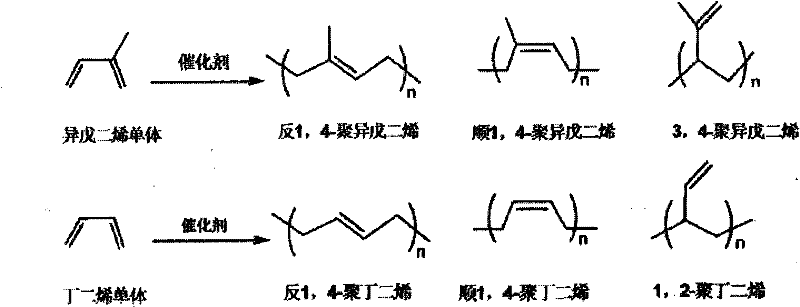
A chromium complex, pincer type technology, applied to catalysts containing pincer chromium complexes, catalyzing isoprene or butadiene trans-1 field, can solve the problems of different polymer ratios, difficulties, etc., and achieve a narrow molecular weight distribution. , the effect of fast polymerization reaction and convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
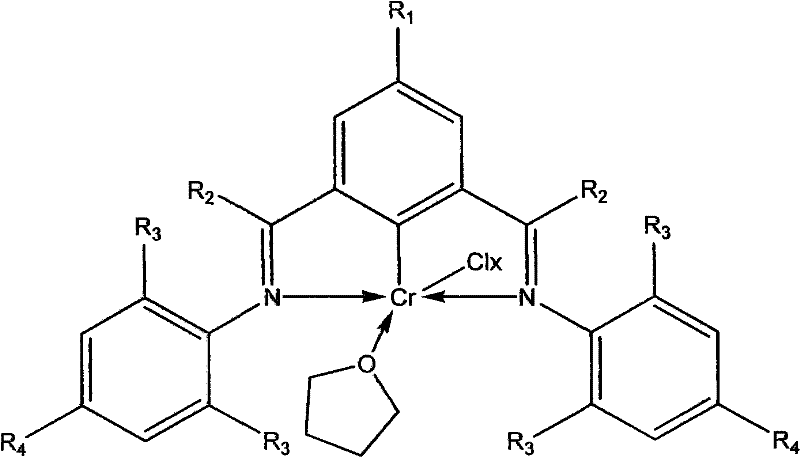
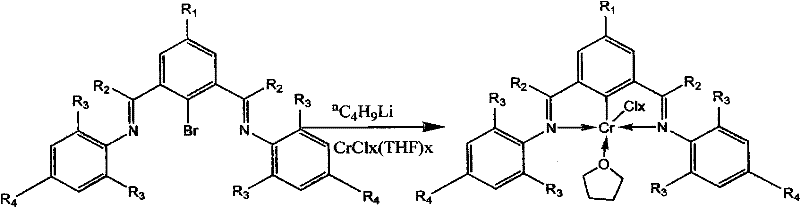
[0037] Example 1 Preparation of clamp-type chromium complex (chromium complex 1)
[0038]
[0039] At -78°C, a hexane solution of butyllithium (1.2mL, 1.88mmol) was slowly added dropwise to 2,6-bis(N-2,6-phenyl)iminobromobenzene (0.6690g , 1.84mmol) in 30mL tetrahydrofuran solution, add CrCl to it after 30 minutes of low temperature reaction 3 (THF) 3 (0.7043g, 1.88mmol), naturally warmed to room temperature and continued to react for 4 hours, the solvent was removed in vacuo, the residue was extracted with toluene, and the toluene solution was concentrated to obtain 0.52 g of bright green crystal complex 1 with a yield of 60%. Elemental analysis, its molecular formula is C 24 h 23 Cl 2 CrN 2 O (%): C, 60.26; H, 4.85; N 5.86.
Embodiment 2
[0040] Example 2 Preparation of clamp-type chromium complex (chromium complex 2)
[0041]
[0042] At -78°C, a hexane solution of butyllithium (0.86mL, 1.35mmol) was slowly added dropwise to 2,6-bis(N-2,6-dimethylphenyl)iminobromobenzene (0.5625g, 1.34mmol) in 30mL tetrahydrofuran solution, add CrCl to it after 30 minutes of low temperature reaction 3 (THF) 3 (0.5148g, 1.37mmol), naturally warmed to room temperature and continued to react for 4 hours, the solvent was removed in vacuo, the residue was extracted with toluene, and the toluene solution was concentrated to obtain 0.50 g of bright green crystal complex 2 with a yield of 71%. Elemental analysis, its molecular formula is C 28 h 31 Cl 2 CrN 2 O (%): C, 62.92; H, 5.85; N, 5.24.
Embodiment 3
[0043] Example 3 Preparation of clamp-type chromium complex (chromium complex 3)
[0044]
[0045] At -40°C, a hexane solution of butyl lithium (1.33 mL, 1.33 mmol) was slowly added dropwise to 2,6-bis(N-2,6-diethylphenyl)iminobromobenzene (0.5773g, 1.21mmol) in 30mL tetrahydrofuran solution, add CrCl to it after 30 minutes of low temperature reaction 3 (THF) 3 (0.5100g, 1.36mmol), naturally warmed to room temperature and continued to react for 4 hours, the solvent was removed in vacuo, the residue was extracted with toluene, and the toluene solution was concentrated to obtain 0.42 g of bright green crystal complex 3 with a yield of 56%. Elemental analysis, its molecular formula is C 32 h 39 Cl 2 CrN 2 O (%): C, 65.08; H, 6.66; N, 4.74.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com