Dynamic kinetic method for resolving secondary alcohol
A dynamic kinetics, secondary alcohol technology, applied in fermentation and other directions, can solve the problems of reduced optical purity and high theoretical yield of products, and achieve great application value and improve performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
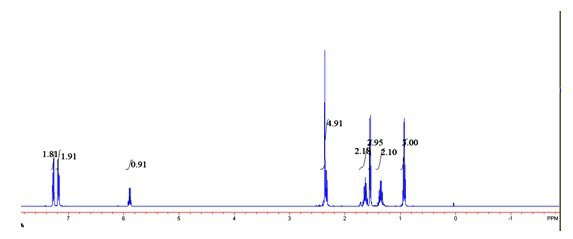
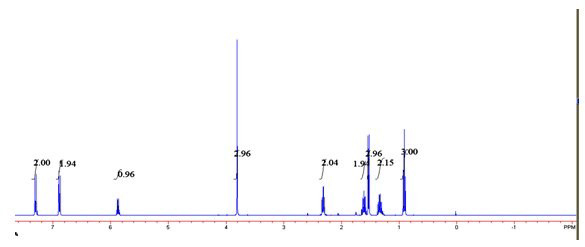
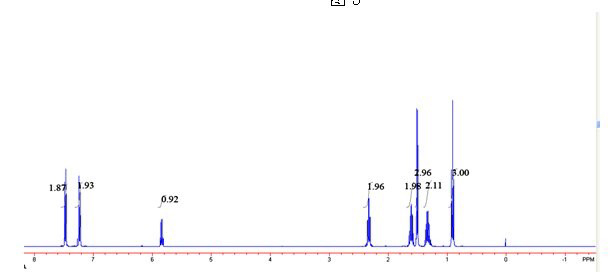
Image
Examples
Embodiment 1
[0023] 1) adding p-chlorophenol, n-valeric acid, dicyclohexylcarbodiimide and 4-dimethylaminopyridine in a molar ratio of 1:1:1:0.03, stirring and reacting for 7 hours, filtering, drying the filtrate, and concentrating, Cross the column to obtain p-chlorophenyl n-valerate as the acyl donor for subsequent use;
[0024] 2) in 2mL of toluene, add phenethyl alcohol and p-chlorophenyl n-valerate in a molar ratio of 1:1, add 10mg / mLCD8604 and 5 mg / mL Novozyme 435 to react for 12 hours, and the reaction temperature is 30° C. to obtain a transformation efficiency. 100% ester with e.e value > 99%.
Embodiment 2
[0026] 1) adding p-chlorophenol, n-valeric acid, dicyclohexylcarbodiimide and 4-dimethylaminopyridine in a molar ratio of 1:2:2:0.05, stirring and reacting for 3 hours, filtering, drying the filtrate, and concentrating, Cross the column to obtain p-chlorophenyl n-valerate as the acyl donor for subsequent use;
[0027] 2) in the toluene of 6mL, add phenylethyl alcohol and p-chlorophenyl n-valerate that the molar ratio is 1:6, add 40mg / mLCD550 and 20mg / mL Novozyme 435 and react for 6 hours, and the reaction temperature is 60 ℃, and obtains transformation efficiency. 100% ester with e.e value > 99%.
Embodiment 3
[0029] 1) add p-chlorophenol, n-hexanoic acid, dicyclohexylcarbodiimide and 4-dimethylaminopyridine in a molar ratio of 1:1.5:1.5:0.05, stir and react for 6 hours, filter, dry the filtrate, concentrate, pass Column, obtain p-chlorophenyl n-hexanoate for subsequent use as acyl donor;
[0030] 2) in 2mL of toluene, add phenethyl alcohol and p-chlorophenyl n-hexanoate with a molar ratio of 1:3, add 20mg / mLCD8604 and 15 mg / mL Novozyme 435 to react for 6 hours, and the reaction temperature is 40° C. to obtain the transformation efficiency. 100% ester with e.e value > 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com