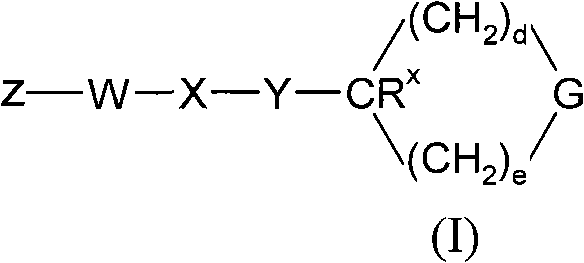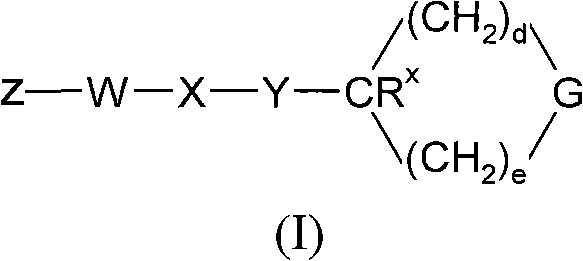Heterocyclic gpcr agonists
A nitrogen heterocycle, heteroaryl technology, applied in the field of GPR119 agonists, can solve the problems of insufficient treatment of dyslipidemia and hyperglycemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
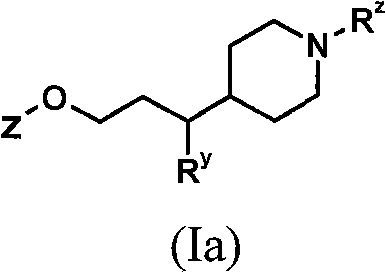
[0138] Processes for the preparation of compounds of general formula (I) as described above also represent a further aspect of the invention.
[0139] As indicated above, the compounds of general formula (I) are useful as GPR119 agonists, eg for the treatment and / or prevention of obesity and diabetes. For such use, the compounds of general formula (I) are usually administered in the form of pharmaceutical compositions.
[0140] The present invention also provides the compound of general formula (I), or a pharmaceutically acceptable salt thereof, for use as a medicine.
[0141] The present invention also provides a pharmaceutical composition comprising a compound of general formula (I) in combination with a pharmaceutically acceptable carrier.
[0142] Preferably, the composition consists of a pharmaceutically acceptable carrier and a non-toxic therapeutically effective amount of the compound of general formula (I) or a pharmaceutically acceptable salt thereof.
[0143] Moreo...
Embodiment 1
[0335] Example 1: tert-butyl 4-{3-[3-fluoro-4-(5-methyltetrazol-1-yl)phenoxy]propyl}piperidine-1-carboxylate
[0336]
[0337] DIAD (335 μL, 1.70 mmol) was added to stirred 3-fluoro-4-(5-methyl-tetrazol-1-yl)phenol (Preparation 5, 150 mg, 773 μmol), tert-butyl 4-(3 -Hydroxypropyl)piperidine-1-carboxylate (207mg, 850μmol) and PPh 3 (264 mg, 1.00 mmol) in THF (7 mL) and the resulting solution was stirred at ambient temperature for 3.5 hours. add additional PPh 3 (80 mg, 309 μmol), stirring was continued at ambient temperature for 1.5 hours, then the reaction mixture was concentrated in vacuo. Purification by RP-HPLC afforded the title compound: RT = 4.13 min; m / z (ES + )=420.14[M+H] + (Method A).
Embodiment 2
[0338] Example 2: 4-{3-[3-fluoro-4-(3-methyl-[1,2,4]oxadiazol-5-yl)phenoxy]propyl}piperidine-1-carboxylic acid Isopropyl ester
[0339]
[0340] NaH (60%, 24.0 mg, 572 μmol, washed with IH) was added to a solution of N-hydroxy-acetamidine (40.0 mg, 630 μmol) in THF (4 mL), and the resulting solution was stirred at ambient temperature for 10 min. Add isopropyl 4-[3-(3-fluoro-4-methoxycarbonylphenoxy)propyl]piperidine-1-carboxylate (Preparation 6, 200 mg, 520 μmol) in THF (4 mL) and The resulting solution was stirred at temperature for 20 hours. with H 2 O quenched the reaction, diluted with EtOAc and washed the organic layer with brine, dried (MgSO 4 ), filtered and concentrated in vacuo. Purification by column chromatography (EtOAc-IH, 1:9 to 1:4) afforded the title compound: RT = 4.09 min; m / z (ES + )=406.10[M+H] + (Method A).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com