Method for producing liposome and method for dissolving cholesterol
A liposome and cholesterol technology is applied in the directions of liposome delivery, preparation of microspheres, preparations for skin care, etc., and can solve the problems of uneven particle size of liposomes, difficulty in preparing liposomes with uniform particle diameters, and the like, achieve the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
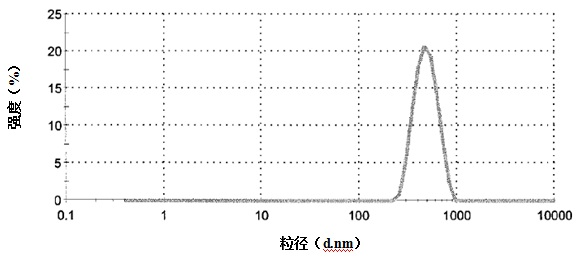
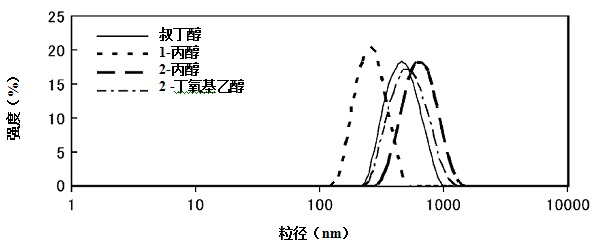
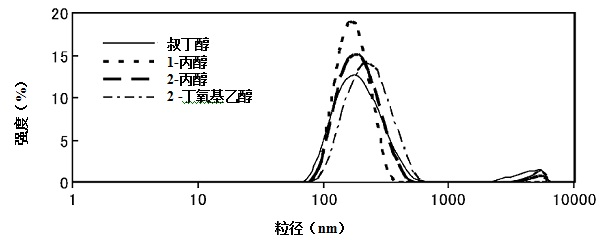
Image
Examples
preparation example Construction
[0032] (2) Preparation of lipid bodies
[0033] The lipid system preparation method described in this method is as long as the heating process of the mixture of a mixture of a mixture of a hydraulic solution containing a water -soluble organic solvent with more than one type of heating, and the cooling process of the cooling mixture after heating process, or the cooling process, or the cooling process can also be.Application method for preparation.More specifically, in order to regulate the particle size of the lipid body, the method of the present invention can also be combined with the ultrasonic dispersing method, extrusion method, French pressure method, and average quality method.
[0034]In the lipid system of the present invention, you can use soybean lecithin, hydrogenation soy lecithin, egg yolk lecithin, phospholipidal alkaline, phospholipidaline, phospholipidal alcoholamine, phospholipidol, phosphorushaliposcin, phosphorushexide phospholipidinSatellite, phospholipidine,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com