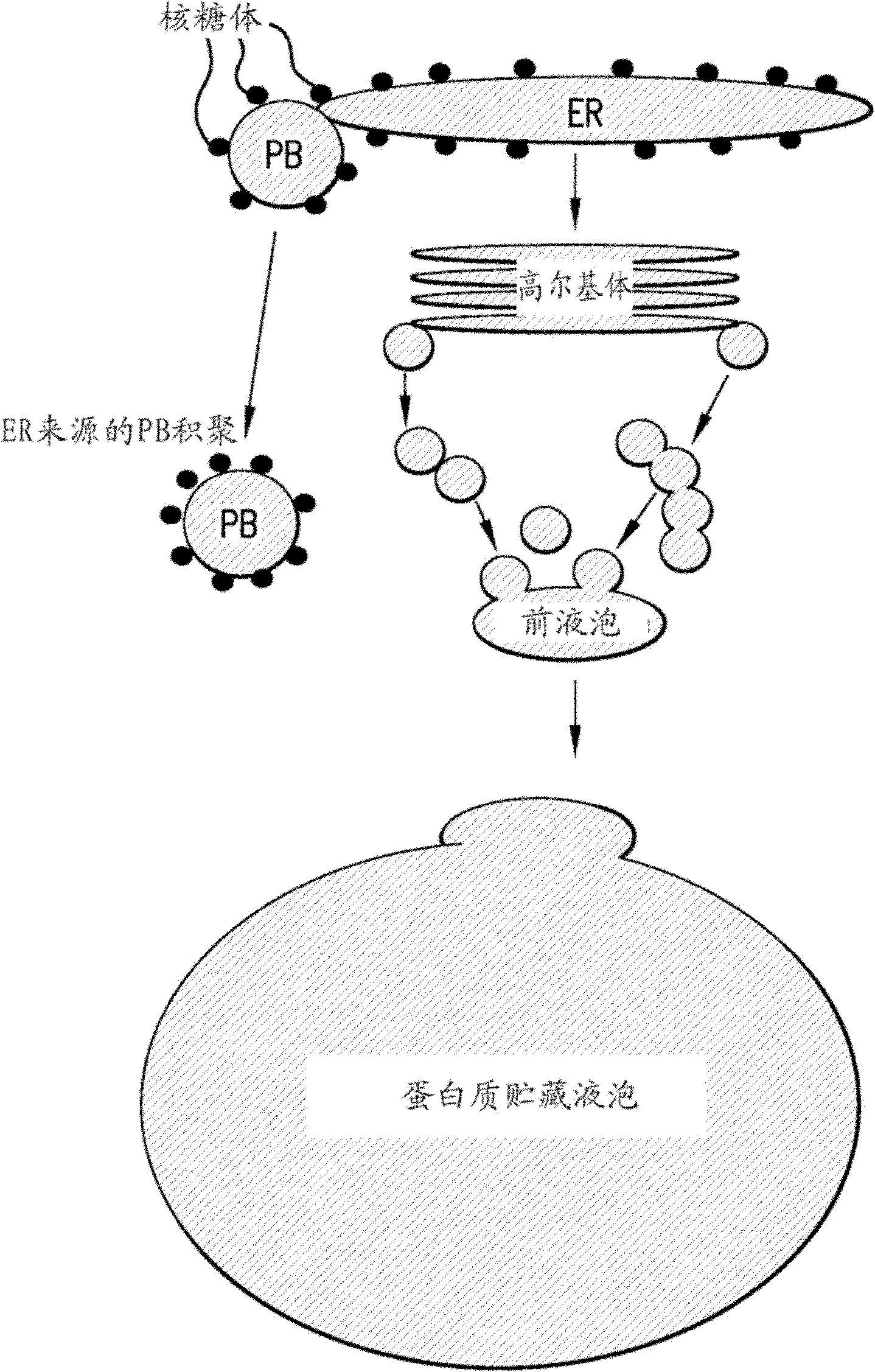
Improved protein production and storage in plants
A protein, heterologous protein technology, applied in the field of plant genetics, can solve the problems of no teaching, reduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
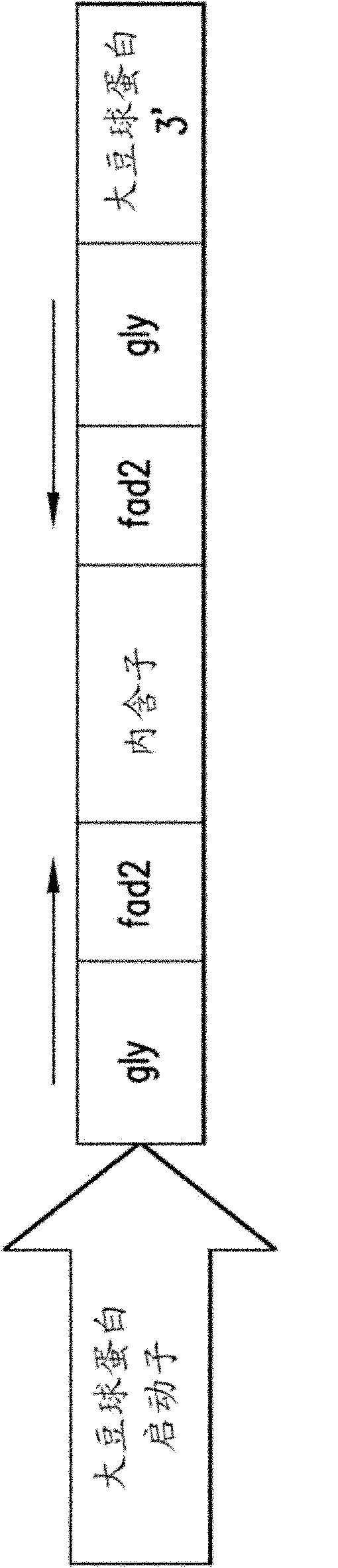
[0210] Generation of storage protein knockdown line "SP-"
[0211] Transformation protocol using gene gun (Parrott et al., 2004, "Transgenic soybean," In: J.E.Specht and H.R.Boerma (eds). Soybeans: Improvement, Production, and Uses, 3rd Edition - Agronomy Monograph No. 16.ASA-CSA- SSSA, Madison, WI.pp 265-302), will be based on figure 2 RNAi constructs designed to suppress storage protein content in seeds were transferred to soybean. Specific simultaneous inhibition of endogenous soybean storage protein and FAD2-1ω-6 was generated by inversion of the sequence specific for this type of open reading frame, which flanks the intron, under the control of the glycinin promoter and 3' terminator RNAi cassette for fatty acid desaturase. The 331 bp region (SEQ ID NO: 55) of the glycinin AlbB2 gene was placed next to the 128 bp region (SEQ ID NO: 57) of the FAD2-1a gene. This 459 bp heterologous DNA was then placed around the intron as an inverted repeat. Introns of synthetic origi...
Embodiment 2
[0215] Protein and oil content in SP-
[0216] The SP-lines were regenerated into soybean plants. The resulting plant growth and fruit set were not significantly different from the control. TO seeds were broken to determine the phenotype, SP-seeds were regrowth and selected two more times to generate a homozygous population. The SP-phenotype was stable in each subsequent generation with no detectable α / α' and β conglycinin subunits and greatly reduced glycinin levels. Oleic acid levels were higher than 94% in SP-seeds, indicating that there was also FAD2 selection marker knockdown.
[0217] The average dry size and weight of 146 mg of dormant seeds of SP- grown in the greenhouse was similar to that of the wild-type (WT) variety "Jack" grown in the greenhouse (mean 163 mg). The total protein and oil content of SP- (40.2%, 19.1%) was similar to that of WT variety "Jack" (37.5%, 20.5%). Thus, this assay demonstrated that knockdown of proteins corresponding to most of the tota...
Embodiment 3
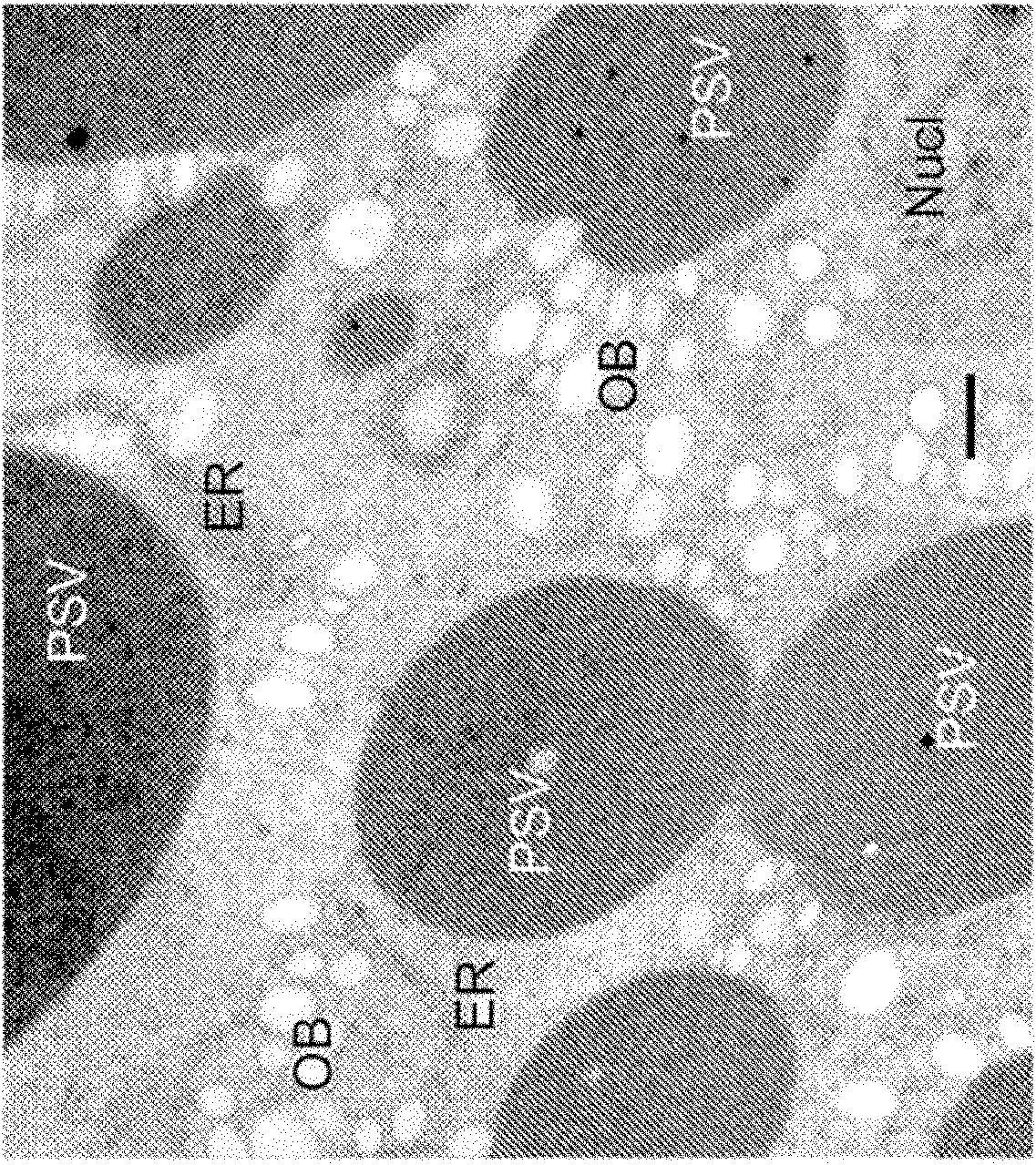
[0219] Electron microscopy and immunogold immunocytochemistry
[0220] Tissue samples were freeze-fixed using a Balzer's high pressure apparatus (Bal-Tech, Principality of Liechtenstein), cryo-displaced with acetone / OsO4, and embedded in epon plastic. Ultrathin sections were stained with saturated uranyl acetate aqueous solution and lead citrate (33 mg / ml), and then observed. Immunocytochemical analysis was then performed. Freeze-fixed parallel samples were then processed by freeze-substitution in the absence of fixative. The displaced samples were transferred to Lowicryl HM-20 resin (which was polymerized by UV light irradiation). Thin sections were labeled with anti-GFP MAb (Clontech) or rabbit polyclonal anti-glycinin previously generated by our laboratory. Sections were indirectly labeled with anti-IgG (rabbit or mouse)-10 nm colloidal gold (Sigma) and then 5% uranyl acetate to increase contrast prior to EM observation. All TEMs were performed with a LEO 912AB microsco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com