Cefcapene pivoxil hydrochloride composition and preparation method thereof
A technology of cefcapene hydrochloride and composition, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, sugar-coated pills, etc., and can solve problems such as unqualified dissolution rate and difficulty in disintegrating cefcapene hydrochloride tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
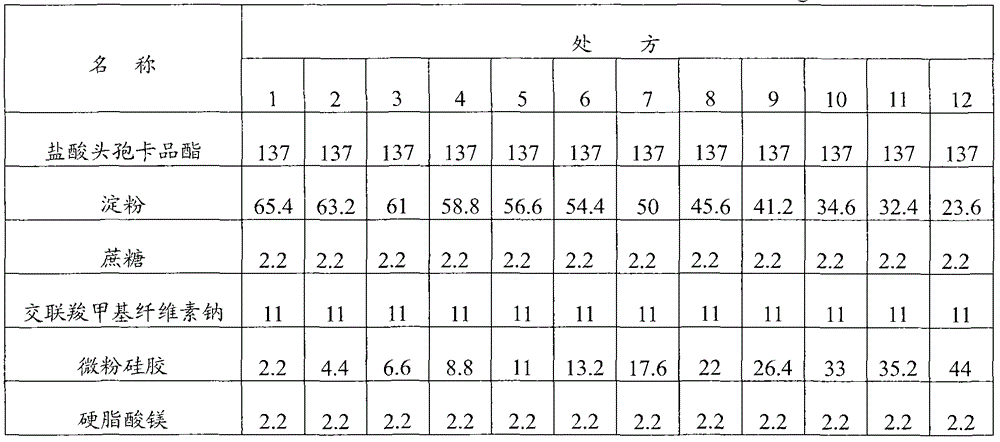
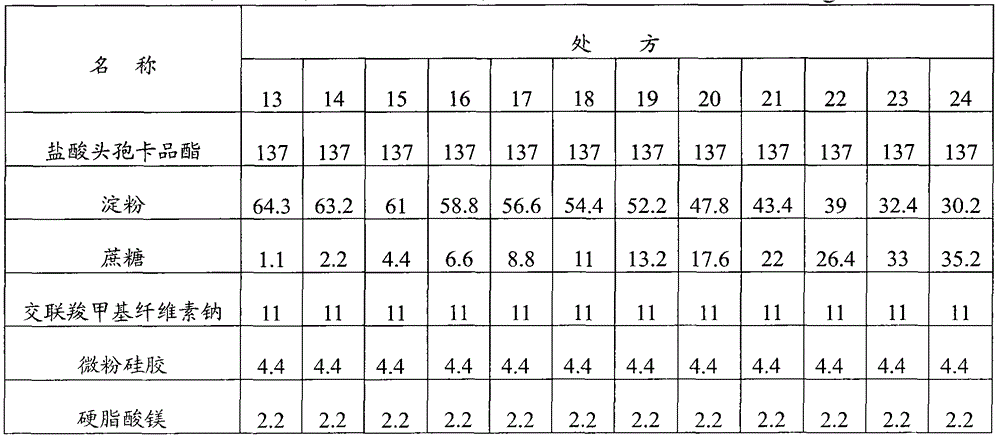
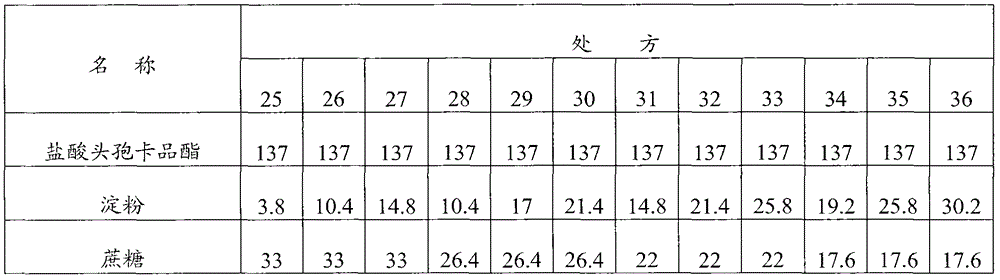
[0093] Embodiment 1: Cefcapene Pixil Hydrochloride Tablets
[0094] Prescription composition (1000 tablets) weight (g) weight percentage (%)
[0095] Cefcapene pivoxil hydrochloride 137 62.3
[0096] Starch 45.6 20.7
[0097] Sucrose 13.2 6
[0098] Croscarmellose sodium 11 5
[0099] Micronized silica gel 11 5
[0100] Magnesium stearate 2.2 1
[0101] Preparation method: first crush and sieve the cefcapene pivoxil hydrochloride raw material to control the particle size to be less than 80 μm, then mix the cefcapene pixil hydrochloride with the micropowder silica gel in the prescribed amount, add the starch, sucrose, and cross-linked carboxymethyl Sodium cellulose, mixed evenly, dry granulated, after granulation, add the prescribed amount of magnesium stearate, mixed evenly, compressed into tablets, coated with film.
[0102] Determination of dissolution: in 1000ml of buffered saline solution with a pH value of 6.8, the paddle method was used to determine the speed of ro...
Embodiment 2
[0103] Embodiment 2: Cefcapene Pixil Hydrochloride Tablets
[0104] Prescription composition (1000 tablets) weight (g) weight percentage (%)
[0105] Cefcapene pivoxil hydrochloride 137 62.3
[0106] Starch 45.6 20.7
[0107] Sucrose 13.2 6
[0108] Croscarmellose sodium 11 5
[0109] Micronized silica gel 11 5
[0110] 30% ethanol solution appropriate amount
[0111] Magnesium stearate 2.2 1
[0112] Preparation method: first crush and sieve the cefcapene pivoxil hydrochloride raw material to control the particle size to be less than 80 μm, then mix the cefcapene pixil hydrochloride with the micropowder silica gel in the prescribed amount, add the starch, sucrose, and cross-linked carboxymethyl Cellulose sodium, mix well, add appropriate amount of 30% ethanol solution, wet granulate, dry at 40°C, add prescription amount of magnesium stearate, mix well, compress into tablets, and coat with film.
[0113] Determination of dissolution: in 1000ml of buffered saline solutio...
Embodiment 3
[0114] Embodiment 3: Cefcapene Pixil Hydrochloride Tablets
[0115] Prescription composition (1000 tablets) weight (g) weight percentage (%)
[0116] Cefcapene pivoxil hydrochloride 137 62.3
[0117] Starch 34.6 15.7
[0118] Sucrose 13.2 6
[0119] Sodium carboxymethyl starch 22 10
[0120] Micronized silica gel 11 5
[0121] Magnesium stearate 2.2 1
[0122] Preparation method: first crush and sieve the cefcapene pivoxil hydrochloride raw material to control the particle size to be less than 80 μm, then mix the cefcapene pivoxil hydrochloride with micropowder silica gel in the prescribed amount, add starch, sucrose, and sodium carboxymethyl starch in the prescribed amount , mixed, dry granulated, after granulated, add the prescribed amount of magnesium stearate, mixed, pressed into tablets, film-coated.
[0123] Determination of dissolution: in 1000ml of buffered saline solution with a pH value of 6.8, the paddle method was used to determine the speed of rotation at 50 r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com