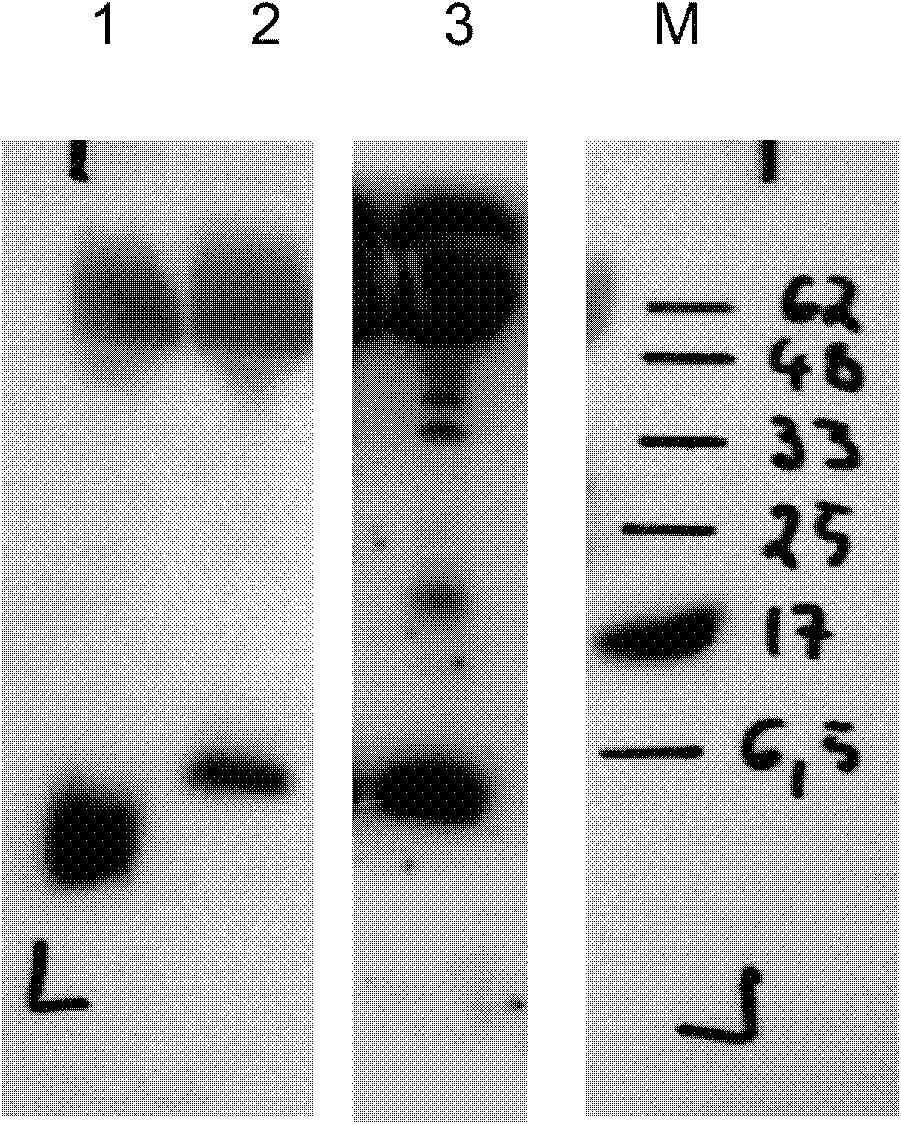Treatment of acute myocardial infarction (AMI) using encapsulated cells encoding and secreting GLP-1 peptides or analogs thereof
A technology of acute myocardial infarction, GLP-1, applied in this field, can solve the problem of not being able to provide, not having an immune response, not being able to obtain AMI or MI treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0231] Preparation of genetic constructs
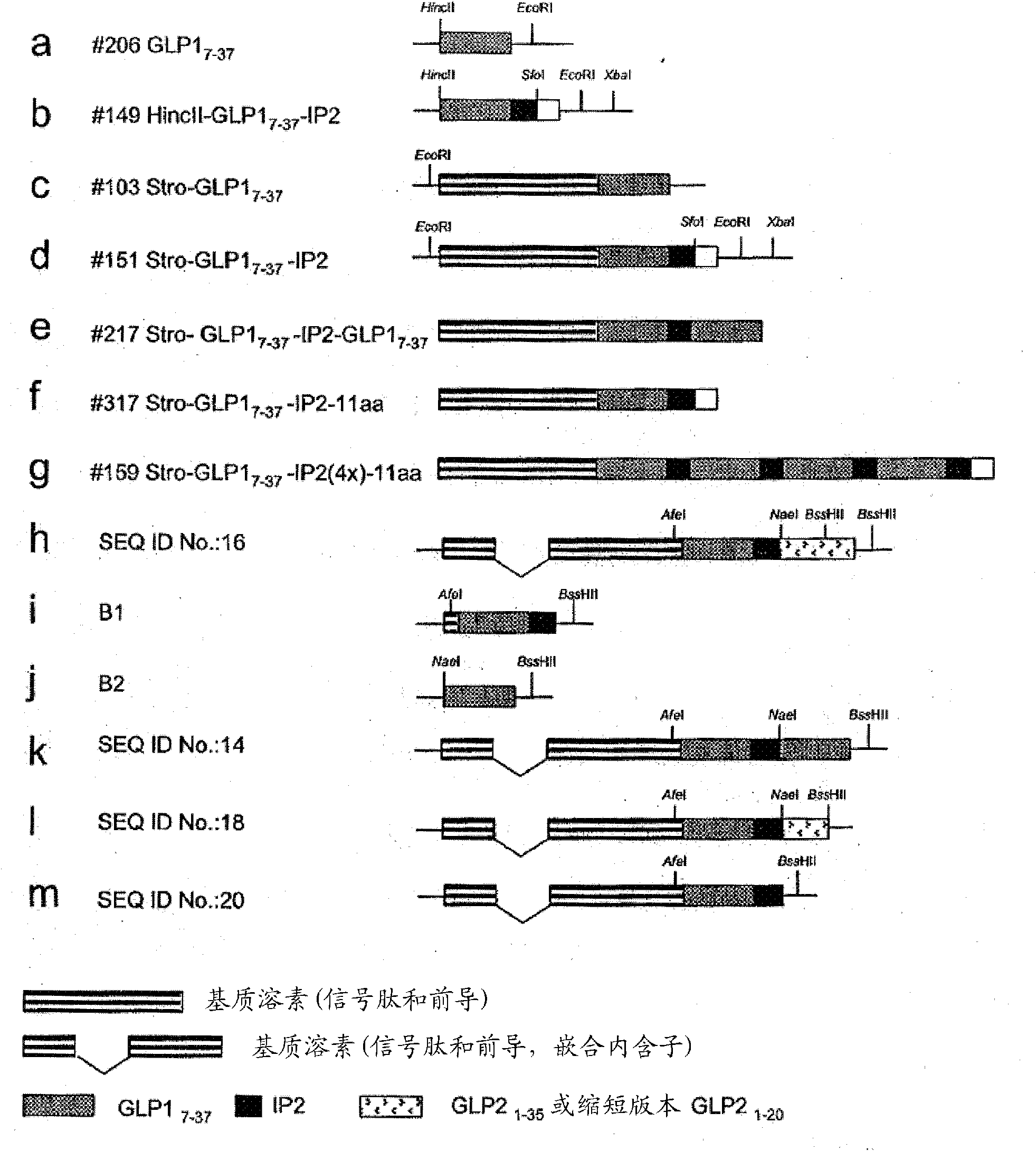
[0232] The coding sequence of GLP-1(7-37) cDNA was synthesized synthetically, such as figure 1 Shown in a, which in turn includes HincII and EcoR1 sites. synthesized separately figure 1 The cDNA indicated in b, as figure 1 Shown in b, which includes the coding sequence for GLP-1(7-37), IP2 and restriction sites for SfoI, EcoRI and XbaI. To direct GLP-1 to the secretory pathway, a heterologous stromelysin 3 signal sequence (Acc No. NM — 005940) was used. Therefore, cDNA encoding the stromelysin signal and leader sequence was amplified by reverse transcriptase PCR from human RNA and compared with figure 1 a or figure 1 The constructs of b are used together to form separately figure 1 c and figure 1 Constructs shown in d.
[0233] Will figure 1 The HincII / EcoRI fragment of the a construct was cloned into figure 1 d sequence in the SfoI site to form the construct figure 1 e. Similarly, the figure 1 The EcoRI fragme...
Embodiment 2
[0237] Mammalian cell transfection, clone selection, and GLP-1 expression
[0238] Source of cells: HEK293 (human embryonic kidney cell line, #ACC 305, DSMZ Cell Culture Collection, Germany), AtT20 (mouse LAF1 pituitary tumor cell line, #87021902, European Cell Culture Collection, UK), hTERT-MSC cells were obtained from Prepared and provided by Prof. Kassem, Odense University Hospital, Denmark.
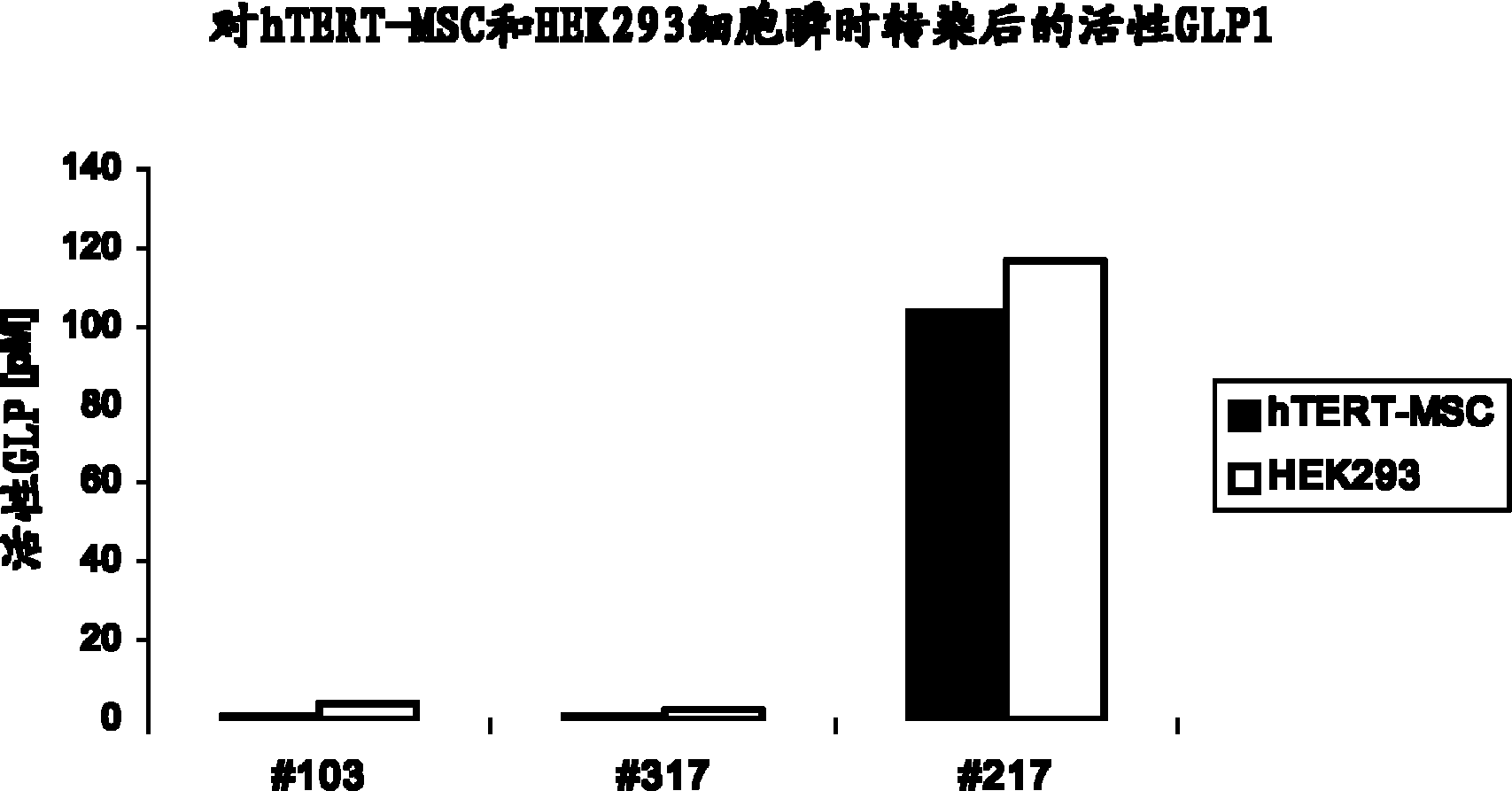
[0239] For transfection 10 6cells, 0.5 μg–2 μg of plasmid DNA with different GLP-1 constructs was used. Constructs were produced as described in Example 1. HEK293 cells were transfected using the standard calcium phosphate co-precipitation method as described in Current Protocols in Molecular Biology (Ausubel et al. 1994ff Harvard Medical School Vol 2., Unit 9.1). AtT20 cells were transfected using FuGene (Roche) as described in Current Protocols in Molecular Biology (Ausubel et al. 1994ff, Harvard Medical School Vol 2., Unit 9.4). Transfection of hTERT-MSC cells was performed usi...
Embodiment 3
[0244] Western blot analysis of GLP-1 peptides secreted by mammalian cells
[0245] The cell culture supernatant from GLP-1 secreting cells was separated by 10%-20% gradient SDS PAGE (120V, 90 minutes), and transferred to PVDF membrane by semi-dry blotting (2.0mA / cm2, 60 minutes) ( Immobilon-P Membrane 0.45 μm Millipore IPVH 00010). After methanol fixation and blocking (3% (w: v) BSA, 0.1% (v: v) Tween-20 in TBS solution), 1 μg / ml anti-GLP-1 antibody (HYB147-12, Antibodyshop ) overnight (o / n) immunoblotting of the membrane. After washing and incubation with 0.02 μg / ml detection antibody (Anti Mouse IgG, HRP conjugated, Perkin Elmer PC 2855-1197) for 4 hours at room temperature, chemiluminescent detection revealed the location of the protein.
[0246] Western blot analysis is shown in image 3 (1: 100 ng of synthetic GLP-1(7-37) dissolved in the supernatant of mock-transfected hTERT-MSC cells; 2: hTERT-MSC cells secreting dimeric GLP-1 from construct #217 ( Clone 79TM217 / 13...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com