Malaria infrared stage DNA vaccine and preparing method thereof
A DNA vaccine and infrared technology, which is applied in the field of biomedicine, can solve the problems that the efficiency and duration of immune protection cannot reach the effective malaria vaccine, and achieve the effect of good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, construction of recombinant plasmid pFLAG-CMV8-gp96NTD / CSP
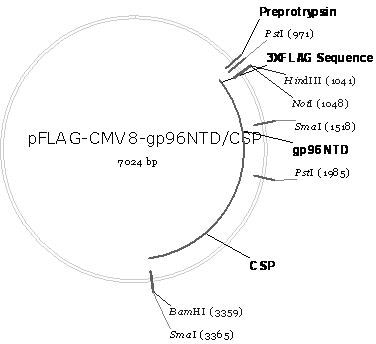
[0035] The schematic diagram of the construction of the recombinant plasmid pFLAG-CMV8-gp96NTD / CSP is as follows figure 1shown. It can be seen from the figure that pFLAG-CMV8-gp96NTD / CSP is the coding gene (nucleotide sequence shown in SEQ ID No.2) of gp96NTD (amino acid sequence shown in SEQ ID No.1) and CSP (amino acid sequence shown in SEQ ID No. ID No.3) coding gene (nucleotide sequence shown in SEQ ID No.4) is inserted in series into the multiple cloning site of the eukaryotic expression vector pFLAG-CMV8 not I and Bam H between Ⅰ and obtained.
[0036] 1. Construction of recombinant plasmid pMD19 simple T / CSP
[0037] 1. Isolation of Plasmodium sporozoites and extraction of total RNA
[0038] The BY265 strain of Plasmodium yoelii was infected with Anopheles stephensi according to the conventional method. On the 15th day after infection, the sporozoites were isolated from the saliva...
Embodiment 2
[0071] Example 2. Construction of recombinant plasmid pFLAG-CMV8-gp96 / CSP (control plasmid)
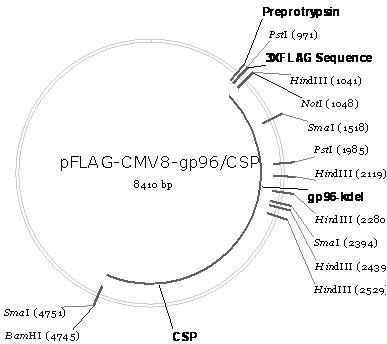
[0072] The schematic diagram of the construction of the recombinant plasmid pFLAG-CMV8-gp96 / CSP is as follows figure 2 shown. It can be seen from the figure that pFLAG-CMV8-gp96 / CSP is the heat shock protein gp96 polypeptide fragment gp96 ΔKDEL (The amino acid sequence is shown in SEQ ID No.9, that is, the key sequence KDEL anchored in the endoplasmic reticulum is removed from the full-length gp96 sequence) The coding gene (nucleotide sequence is shown in SEQ ID No.10) and The coding gene (nucleotide sequence shown in SEQ ID No.4) of CSP (amino acid sequence shown in SEQ ID No.3) is inserted into the multiple cloning site of eukaryotic expression vector pFLAG-CMV8 after tandem not I and Bam H between Ⅰ and obtained.
[0073] 1. Construction of recombinant plasmid pFLAG-CMV8-gp96
[0074] According to the gene sequence of gp96 (GenBank accession number U01153), PCR primers wer...
Embodiment 3
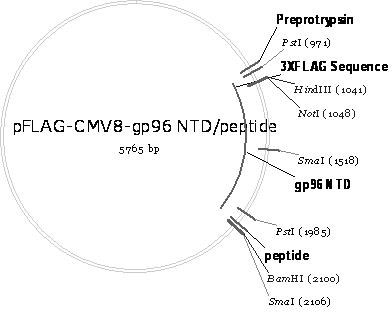
[0079] Example 3. Construction of recombinant plasmid pFLAG-CMV8-gp96NTD / peptide (control plasmid)
[0080] The schematic diagram of the construction of the recombinant plasmid pFLAG-CMV8-gp96NTD / peptide is as follows image 3 shown. It can be seen from the figure that pFLAG-CMV8-gp96NTD / peptide combines the coding gene (nucleotide sequence shown in SEQ ID No.2) of gp96NTD (amino acid sequence shown in SEQ ID No.1) with CSP-specific CD8 + T-cell epitope peptide (amino acid sequence shown in SEQ ID No.13) encoding gene (nucleotide sequence shown in SEQ ID No.14) was inserted into the multiple cloning site of eukaryotic expression vector pFLAG-CMV8 after tandem Bgl II and Bam H between Ⅰ and obtained.
[0081] 1. Encoding CSP-specific CD8 + Annealing of T cell epitope peptide oligonucleotides
[0082] Dissolve the CSP-specific CD8 with 1×T4 DNA ligase buffer + The sense and antisense chain oligonucleotides of T cell epitope peptide were lyophilized to a final concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com