Synthetic method for sucralose
A technology for sucralose and a synthesis method, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of long cycle, difficult purification, low conversion rate, etc., and achieves ingenious methods, mild crystallization, and reduced cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
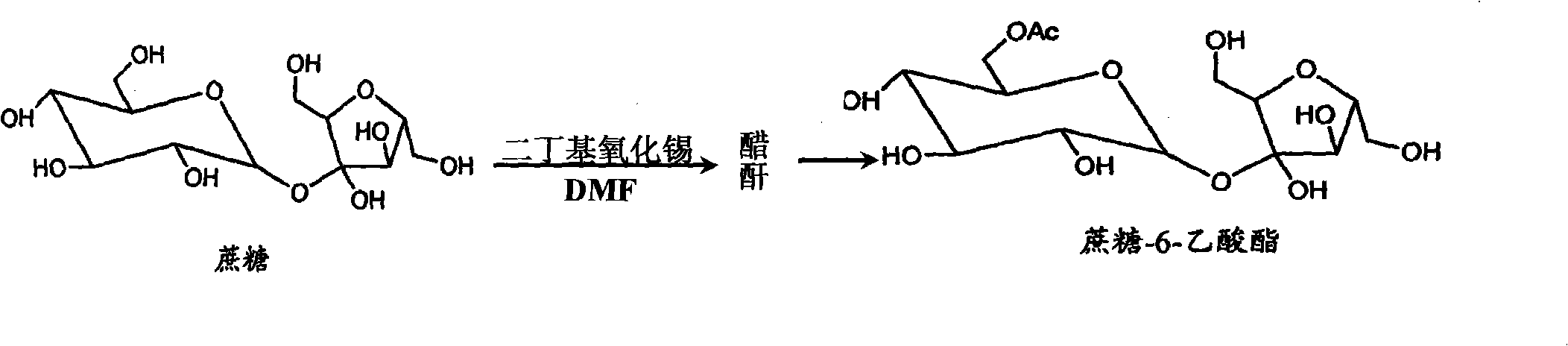
[0013] Example 1: Add 100g of sucrose to 300ml of N,N-dimethylformamide, heat to 80°C to dissolve, cool down to 60°C, then add 75g of dibutyltin oxide and stir, add 250ml of cyclohexane to reflux for dehydration reaction, When the dehydration amount reaches 30ml, lower the temperature to -5°C, start to add 40g of acetic anhydride dropwise, and finish the drop within 20 minutes. Organotin was extracted four times, 300ml each time, and the first cyclohexane extract was used to recover organotin, and the 2, 3, and 4 times were applied in sequence; after separating the cyclohexane layer, a light red sucrose-containing 6-ester N,N-dimethylformamide solution, and then add 250ml of cyclohexane to the solution, reflux dehydration at 85-90°C, when the dehydration amount reaches 45-55ml, cool down to stop the reflux, and drop to room temperature Finally, use a separatory funnel to divide the cyclohexane layer to obtain 320ml of anhydrous sucrose-6-ester solution. Our acylation reaction ...
example 2
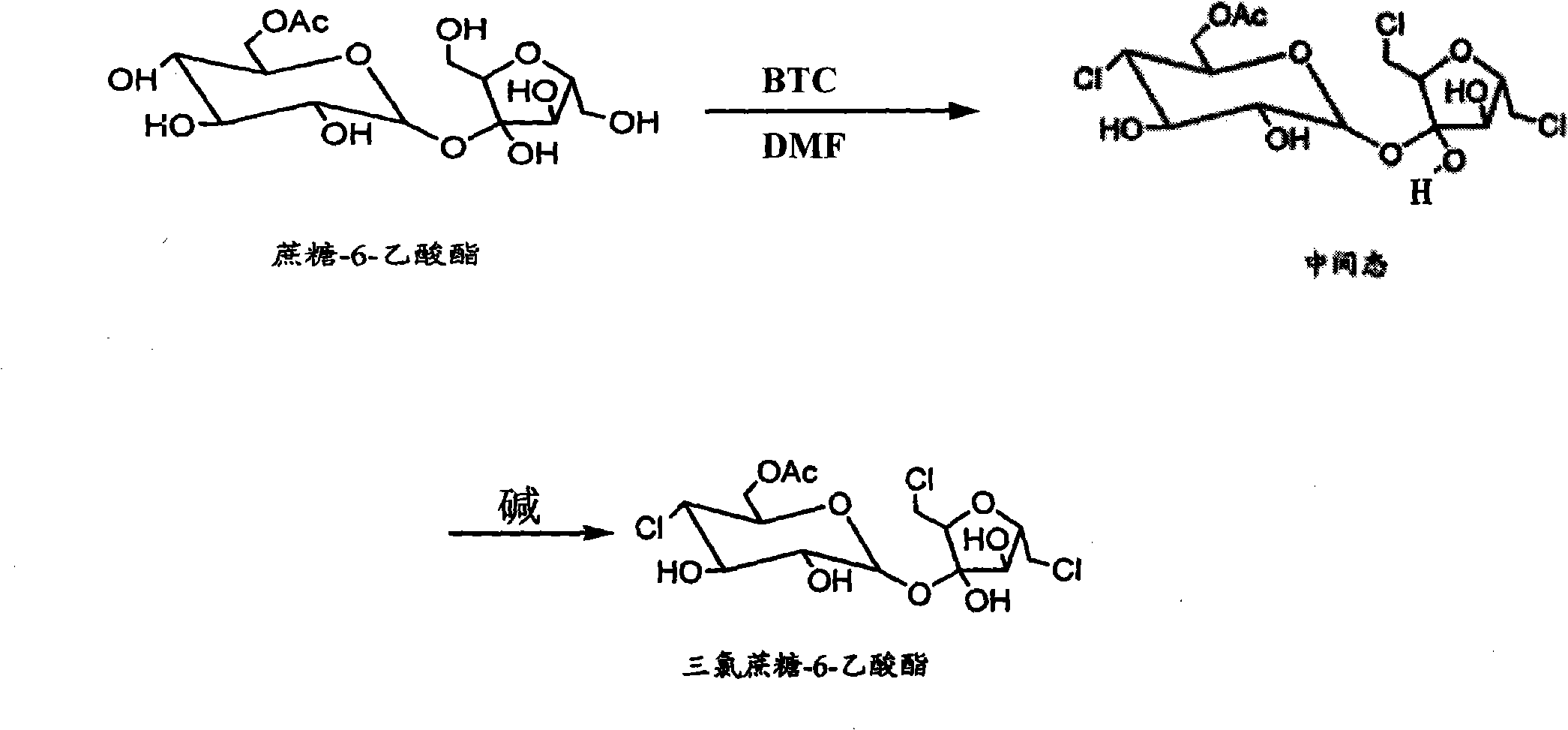
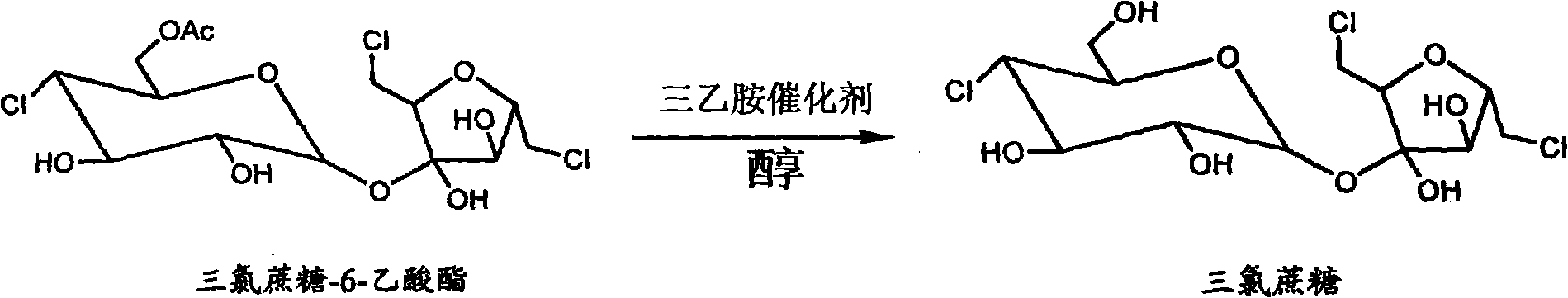
[0014] Example 2: Heat 100g of sucrose to 85°C and dissolve it in 500ml of N,N-dimethylformamide, then cool down to 65°C, then add 90g of dibutyltin oxide and stir, add 500ml of cyclohexane to reflux for dehydration, when When the amount of dehydration reaches 35ml, cool down to 0°C, start to add 60g of acetic anhydride dropwise, usually within 50 minutes, then keep it at 0°C for 30 minutes, add 10ml of water to stop the reaction, then take cyclohexane and divide it into four Extract the organotin twice, 300ml each time, and separate the cyclohexane layer to obtain a light red N,N-dimethylformamide solution containing sucrose-6-ester, and then add 250ml of cyclohexane to the solution , when the dehydration amount reaches 45-55ml, cool down and stop reflux, and then use a separatory funnel to separate the cyclohexane layer after cooling down to room temperature to obtain 510ml of anhydrous sucrose-6-ester solution for the next step of chlorination; first prepare vissmer Reagent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com