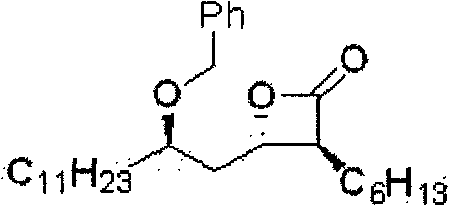
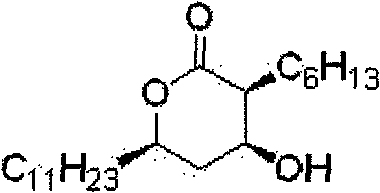
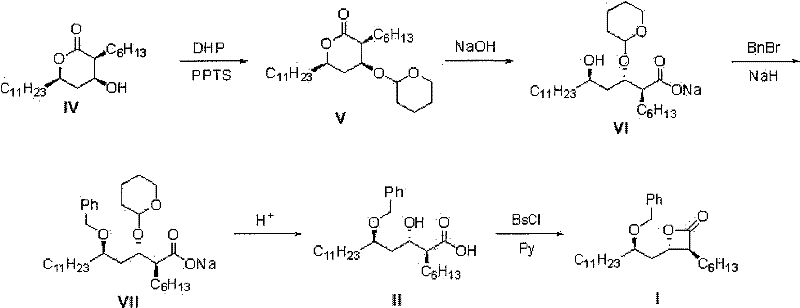
Method for preparing (3S,4S)-4-((R)-2-(benzyloxy)tridecyl)-3-hexyl-2-oxetanone and novel intermediate used therefor
一种杂环丁烷酮、十三烷基的技术,应用在羧酸盐制备、化学仪器和方法、有机化合物的制备等方向,能够解决低总收率等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Preparation of (2S, 3S, 5R)-3,5-dihydroxy-2-hexyl-sodium hexadecanoate (compound of formula III)-(1)
[0067]
[0068] Dissolve 25.5 g of (2S, 3S, 5R)-3-hexyl-4-hydroxy-6-undecyltetrahydropyran-2-one in 178.5 ml of methyl-tert-butyl ether, and add 90 ml of 2N NaOH solution was added, and the resulting solution was stirred for 3 hours while maintaining the temperature of the mixed solution at 50°C. The reaction mixture was then kept at rest until phase separation occurred, its aqueous layer was removed and the remaining organic phase was washed twice with 100 ml portions of brine. The organic solution thus obtained was dried over anhydrous sodium sulfate, filtered and distilled under reduced pressure to remove the solvent to obtain 28.4 g of the title compound as an oil (yield: 100%).
[0069] 1 H-NMR, 300 MHz (CD 3 OD, ppm): δ0.89(dd, 6H, J=5.8, 1.2Hz), 1.24~1.80(m, 32H), 2.102.49(m, 2H), 3.72~3.87(m, 1H)
Embodiment 2
[0070] Example 2: Preparation of (2S, 3S, 5R)-methylbenzylamine salt of (2S, 3S, 5R)-5-benzyloxy-2-hexyl-3-hydroxyhexadecanoic acid (compound of formula II)-(1)
[0071]
[0072] Dissolve 98 ml of sodium (2S,3S,5R)-3,5-dihydroxy-2-hexyl-hexadecanoate obtained in Example 1 above in a mixture of 98 ml of tetrahydrofuran and 328 ml of methyl-tert-butyl ether solvent. The reaction mixture was cooled to 0°C, and 7.19 g of sodium hydroxide and 30.7 g of benzyl bromide were sequentially added to the mixed solution. The temperature of the reaction mixture was then raised to 60° C. and the reaction mixture was refluxed for 5 days (step (a)). After cooling the reaction mixture to room temperature, its pH was adjusted to 1, and the solution was stirred at room temperature for 3 hours. Subsequently, the pH of the reaction mixture was adjusted to 4, and the mixed solution was kept still until phase separation occurred. The aqueous layer was removed and the remaining organic phase was...
Embodiment 3
[0075] Example 3: Preparation of (2S, 3S, 5R)-3,5-dihydroxy-2-hexyl-sodium hexadecanoate (compound of formula III)-(2)
[0076] 300 g of (2S,3S,5R)-3-hexyl-4-hydroxy-6-undecyltetrahydropyran-2-one were dissolved in 1.8 liters of methyl-tert-butyl ether. 9 L of 2N NaOH solution was added to the reaction mixture and the temperature of the reaction mixture was slowly raised, and its aqueous layer was separated and removed from the reaction mixture. The organic layer thereof was separated from the remaining reaction mixture and washed with a 450 ml portion of saturated brine, and the solvent was removed under reduced pressure. 900 ml of toluene was added to the residue and the solvent and water were removed by azeotropic distillation under reduced pressure. To the residue were added 1.8 liters of heptane and 1.8 liters of methyl-tert-butyl ether, and the temperature of the reaction mixture was raised to 40°C. Subsequently, the reaction mixture was cooled at room temperature and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com