Application of 2-arylmorpholine and salt thereof serving as insecticide
An aryl morpholine salt and aryl morpholine technology are applied in the application field of 2-aryl morpholine and its salt as the preparation of pesticides, and can solve the problems of no research and development reports on the application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0018] Preparation of embodiment 12-aryl morpholines and salts thereof
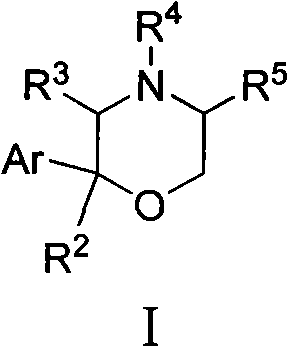
[0019] According to literature [Organic Chemistry, 2004, 24(8): 902-905; Applied Chemistry, 2005, 22(3): 343-345; Journal of Hunan University, 2004, 31(4): 11-14; 2005, 32( 4): 72-76] method to prepare 2-arylmorpholine and its salt (I).
[0020]
[0021] Among them, R 2 Selected from: H, OH or OR 1 , where R 1 Selected from: C 1 ~C 2 Alkyl, C 3 ~C 4 Straight chain alkyl or branched chain alkyl; R 3 From: H, C 1 ~C 2 Alkyl, C 3 ~C 4 Straight chain alkyl or branched chain alkyl; R 4 From: H, C 1 ~C 2 Alkyl, C 3 ~C 4 Straight chain alkyl or branched chain alkyl, hydroxyethyl, Ar'CH 2 , wherein Ar' is selected from: phenyl, 4-methoxyphenyl, 4-fluorophenyl, 4-chlorophenyl, 4-bromophenyl, 4-nitrophenyl, 4-aminophenyl, 4 -Acetamidophenyl, 2-methanesulfonylaminophenyl, 3-methanesulfonylaminophenyl, 4-methanesulfonylaminophenyl, 4-trifluoromethylphenyl, 4-isobutylphenyl or 6-methoxy-2-napht...
Embodiment 22
[0022] Example 22-Arylmorpholine or its salts are tested for poisonous activity against armyworm, broad bean aphid and cotton spider mite
[0023] 1 test target
[0024] Armyworm (My, thimna sepatara) is a sensitive strain that has been raised on fresh corn leaves for many years; the test insects are 3rd instar larvae; broad bean aphid (Aphis fabae) is a sensitive strain that has been raised on broad bean seedlings indoors for many years, and the test insects are 3-day The cotton spider mite (Tetranychus urticae) is a sensitive strain that has been raised indoors with broad bean seedlings for many years. The test insects were healthy adult mites.
[0025] 2 culture conditions
[0026] The culture conditions of the tested target and the target after the test are temperature 25±5° C., relative humidity 65±5%, and photoperiod 12 / 12h (L / D).
[0027]3 Test drug (original drug): 2-arylmorpholine or its salt.
[0028] 4 Preparation of the original drug: use an electronic balance ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com