Peptide for preventing or treating liver damage
A technology for liver injury and acute liver injury, applied in the direction of peptides, peptide/protein components, medical preparations containing active ingredients, etc., can solve problems such as liver cell necrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0066] The preparation of embodiment 2 peptide conjugates
[0067] Peptide A was cross-linked with bovine serum albumin (BSA) to form a conjugate by the glutaraldehyde method. The specific conjugation process is as follows: Take 1 mg of the peptide A synthesized in Example 1 and dissolve it in 0.5 ml of PBS (pH7.4, 0.02mol / L); take 4.5 mg of BSA and dissolve it in 4.5 ml of PBS (pH7.4, 0.02 mol / L). Mix the above peptide A and BSA solutions, then slowly add 1 ml of 0.1% glutaraldehyde, and let the cross-linking reaction proceed for 12 hours at room temperature in the dark. Then glycine solution (1 mol / L) was slowly added to stop the reaction, followed by dialysis with PBS (pH 7.4, 0.02 mol / L) overnight and freeze-drying. The obtained cross-linked product of peptide A and BSA was named as peptide conjugate A.
Embodiment 3
[0068] Example 3 Peptide Reactivity Research with Hepatitis C Patient's Serum
[0069] 3.1 Serum source
[0070] Anti-HCV antibody positive serum: Randomly obtained from hospitalized patients with HCV infection in the 302 Hospital of the People's Liberation Army from 2000 to 2001.
[0071]Control serum: taken from healthy blood donors, and the results of laboratory tests showed that all indicators were normal.
[0072] Hepatitis B (HBV) patient serum: selected from patients inpatients with hepatitis B in the 302 Hospital of the People's Liberation Army, all of which were positive for hepatitis B virus surface antigen in laboratory tests.
[0073] 3.2 The method of detecting serum reactivity by indirect ELISA method
[0074] According to conventional indirect ELISA technology, DG3022 enzyme-linked immunoassay instrument (purchased from Perkin Elmer) was used to detect the reactivity of various peptides of the present invention with various sera. Add 10 μg each of peptide A, ...
Embodiment 4
[0079] Example 4 Peptide Immunogenicity in Mice and Research on Cytokine in Serum
[0080] 4.1 Experimental animals
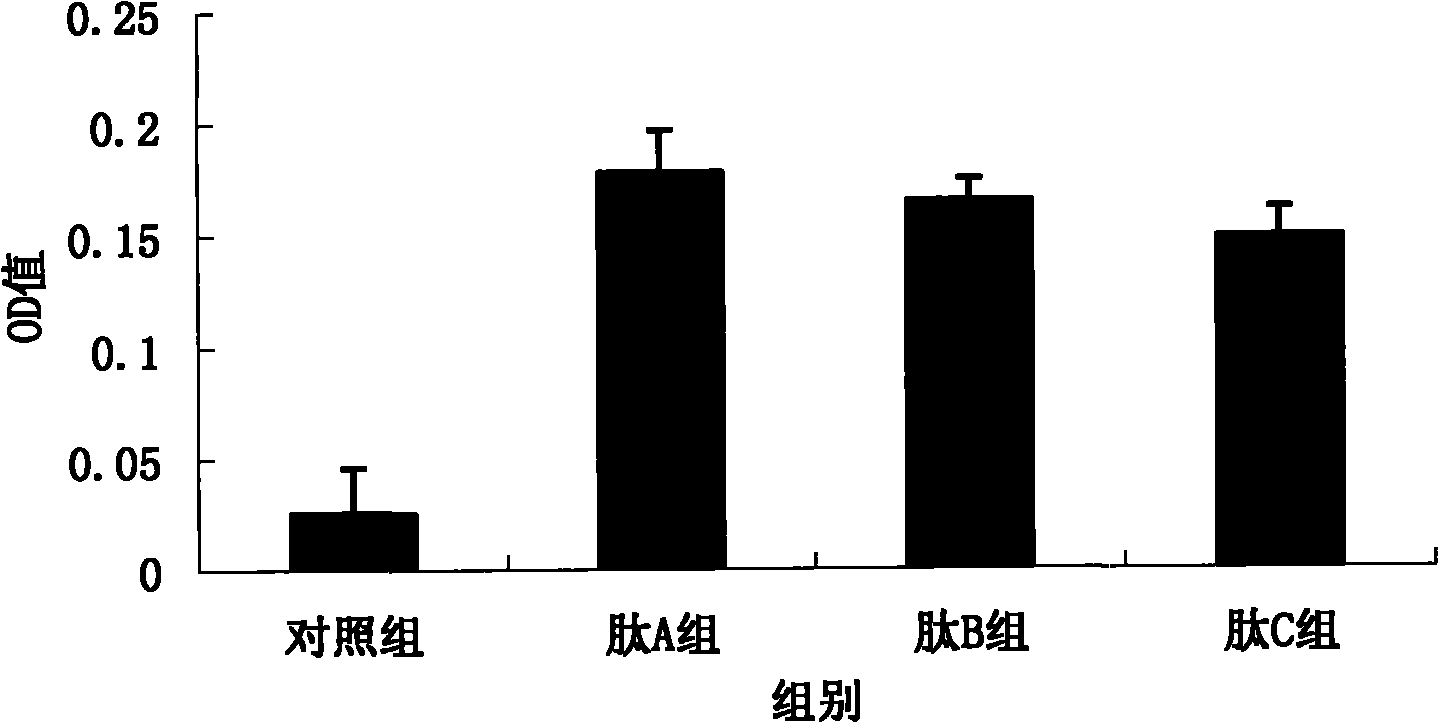
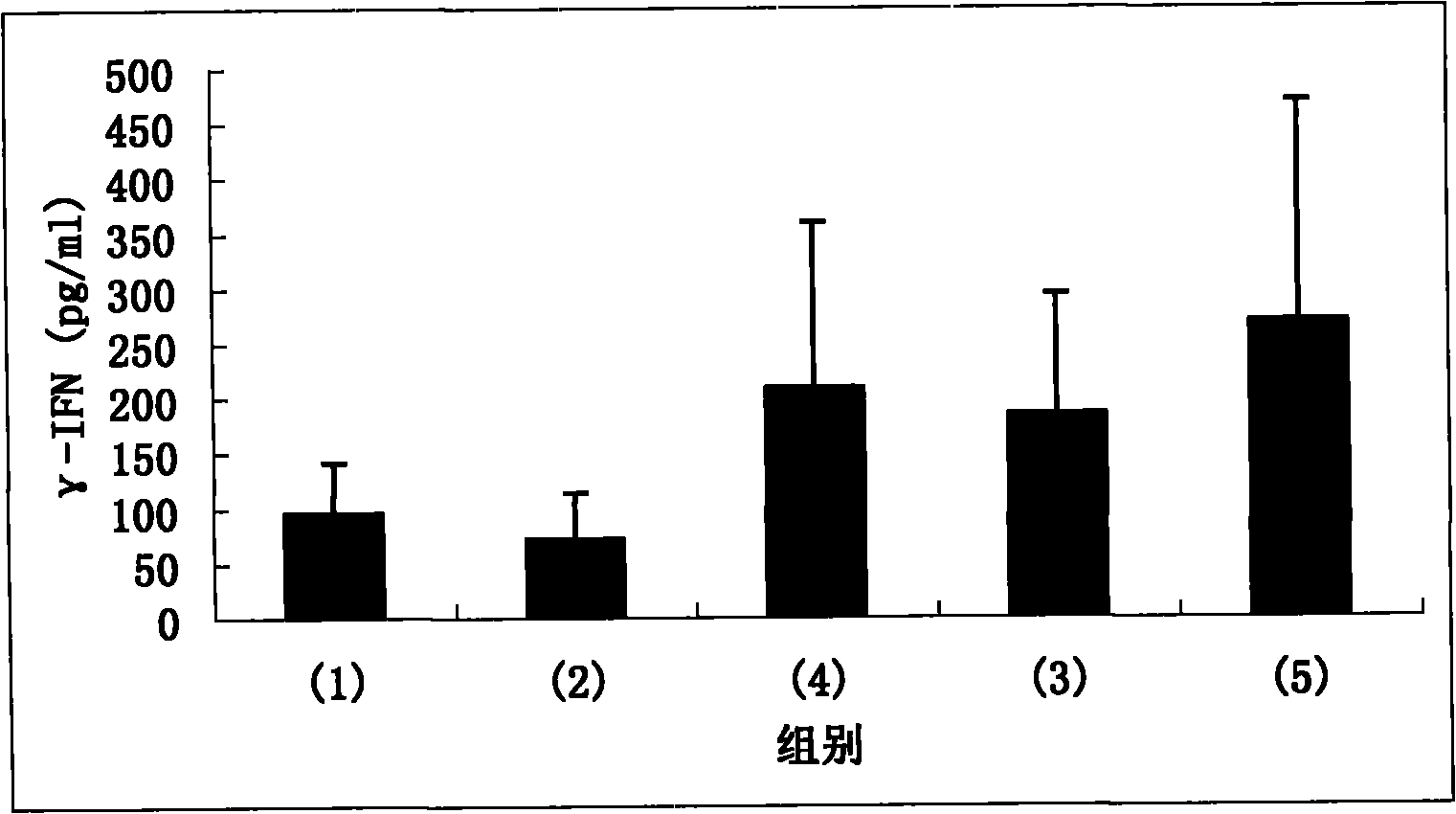
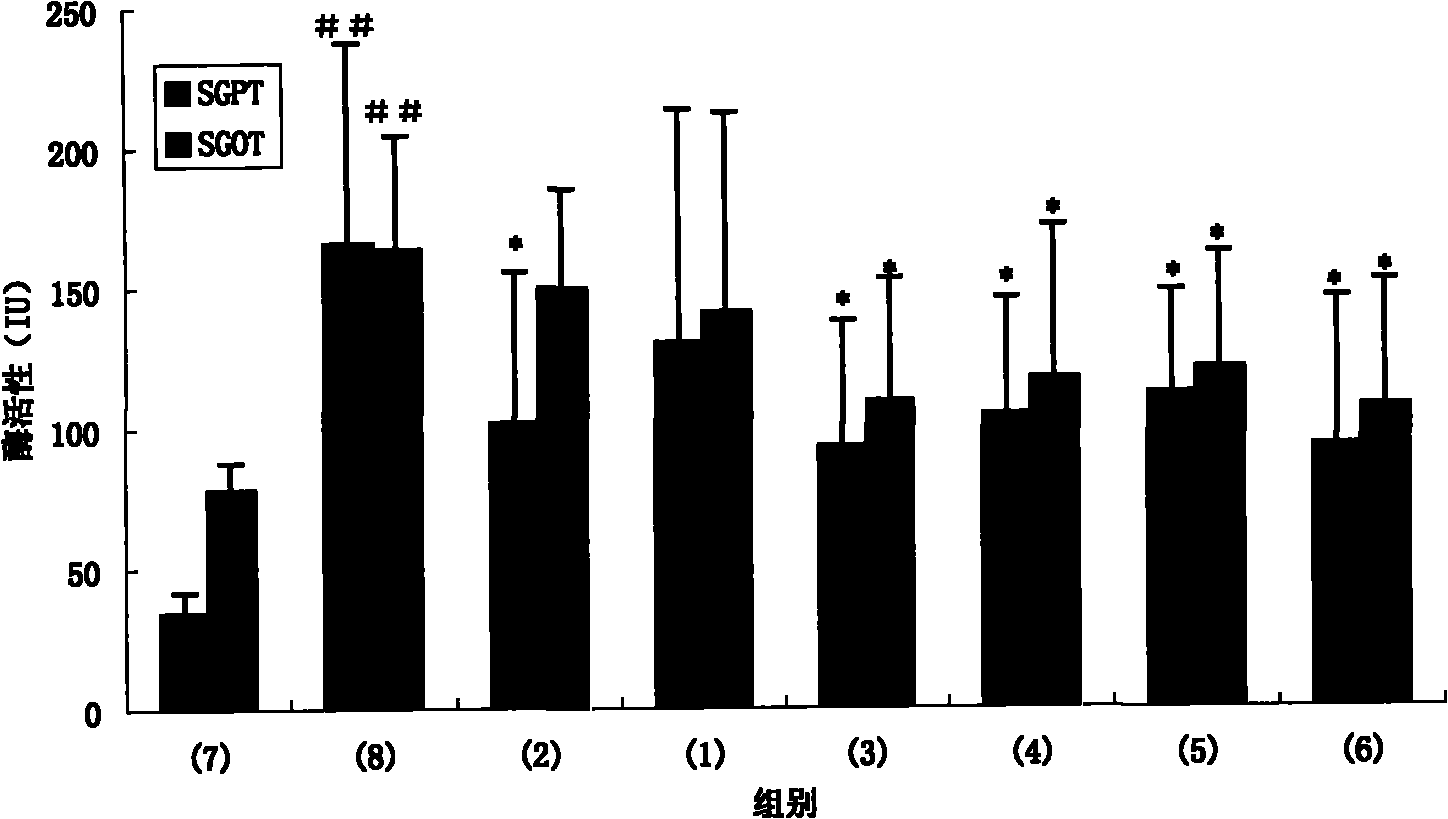
[0081] BALB / c mice (all male, 6 weeks old, purchased from the Experimental Animal Center of the Academy of Military Medical Sciences, Beijing) were taken and divided into the following 4 groups, 5 mice in each group: (1) control group; (2) peptide A group ( (immunized with peptide A); (3) peptide B group (immunized with peptide B); (4) peptide C group (immunized with peptide C).
[0082] 4.2 Immunization method
[0083] The peptides (100 μg / μl) used in each group were mixed with 50 μl of complete Freund’s adjuvant (GIBCOBRL company) in equal volumes, fully mixed with a micro-stirrer, and injected into the foot pads of mice, thus completing the first treatment of mice. Immunization. Booster immunization was carried out 14 days later, 50 μl each of the peptide (100 μg / μl) and Freund’s incomplete adjuvant (GIBCOBRL) used in each group were immunized in the same...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com