Selective electrolysis hydrogenation and dechlorination method for chlorinated organic matter
A chlorinated organic compound, hydrodechlorination technology, applied in electrolysis process, organic chemistry, electrolysis components, etc., can solve problems such as increased energy consumption, graphite influence, fragmentation, etc., to reduce reaction energy consumption and avoid foaming. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 active silver electrode
[0021] In a 250mL beaker, sieve silver was used as the working electrode, with an apparent size of 0.05 cm × 5 cm × 5 cm, graphite with the same apparent size as the anode, and the cathode and anode were separated by 2 cm. The electrolytic solution is 200mL of 2.9wt% sodium chloride+2wt% sodium hydroxide aqueous solution, and the electrolytic solution remains static. Then the silver electrode is oxidized by direct current, the current density is 50mA / cm 2 , after the electrode potential drops to 0.9 volts, the polarity is reversed to reduce the silver electrode, and the current density is 100mA / cm 2 After the electrode potential rises to -0.8 volts, the power supply is stopped, the reaction temperature is controlled at 25°C, and the cell voltage is 2.5-3.5V. Take out the silver electrode and immerse it in deionized water for later use.
Embodiment 2
[0022] Example 2 Preparation of 3,6-dichloropicolinic acid by electrolysis of 3,4,5,6-tetrachloropicolinic acid
[0023] In a 1000 mL beaker, 78 g of 3,4,5,6-tetrachloropicolinic acid (3,4,5,6-TCP) (98% content) was dissolved in 0.5M Na 2 CO 3 in aqueous solution (1000 mL). The active silver electrode prepared with embodiment 1 is negative electrode, and ruthenium titanium electrode is anode (its surface is made up of ruthenium dioxide and titanium dioxide, wherein the content of ruthenium is 15g / m 2 ;Titanium content is 15g / m 2 ;), electrolysis was carried out at 50°C (the current was 5A), and after 2 hours of electrolysis, the electrolysis was stopped for 15 minutes to restore the electrode activity. After the electrode activity was restored, the electrolysis was continued for 2 hours (the current was 5A), and then the electrolysis was stopped for 15 minutes to restore the electrode activity. After the electrode activity is restored again, add 10 grams of sodium hydroxid...
Embodiment 3~ Embodiment 5
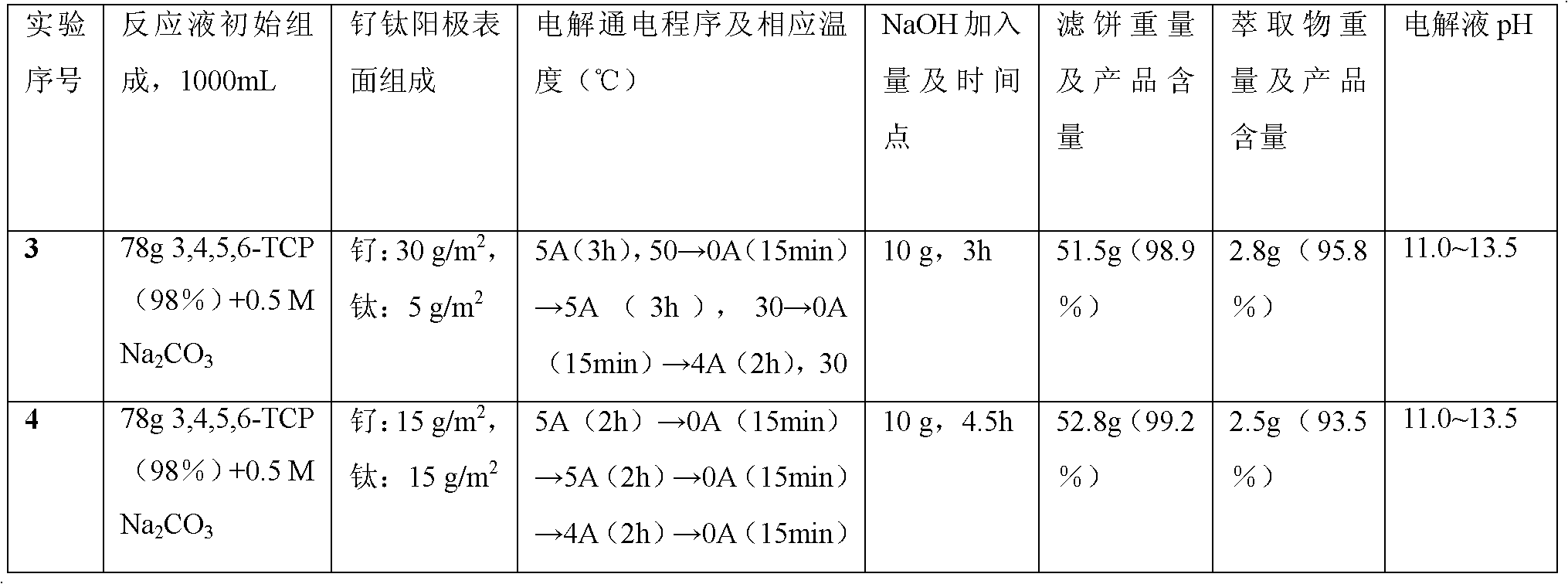
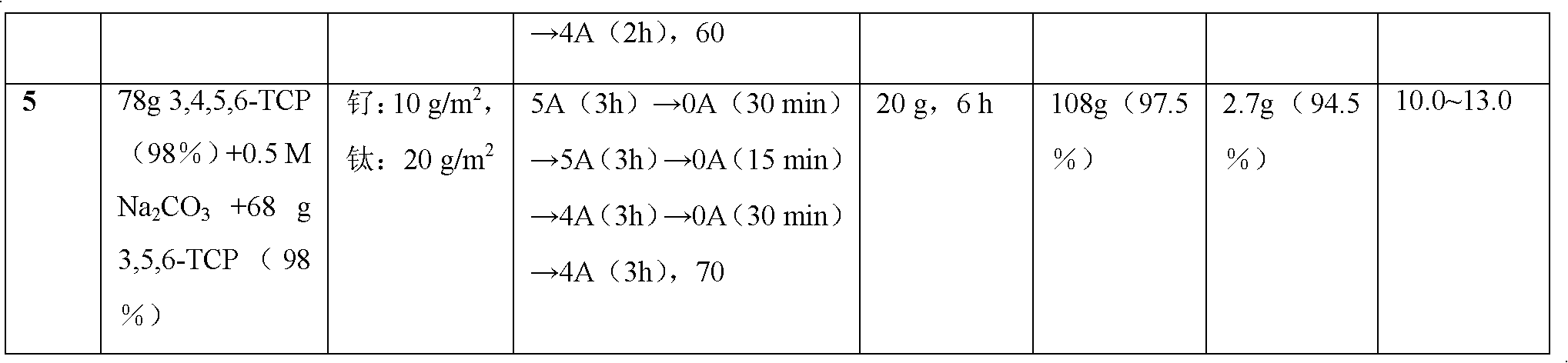
[0025] According to the experimental parameters in Table 1:
[0026] Table 1 1000mL scale electrolytic dechlorination to prepare 3.6-dichloropicolinic acid
[0027]
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com