Catalyst component and catalyst used for olefin polymerization, and application thereof
A technology of olefin polymerization and catalyst, which is applied in the field of olefin polymerization and can solve problems such as ligand compounds that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
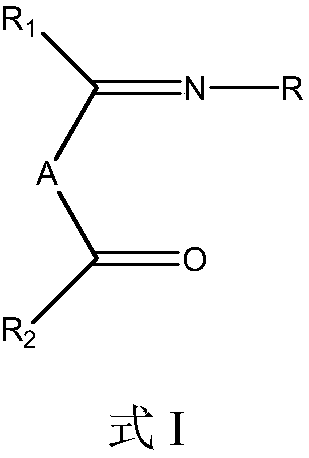
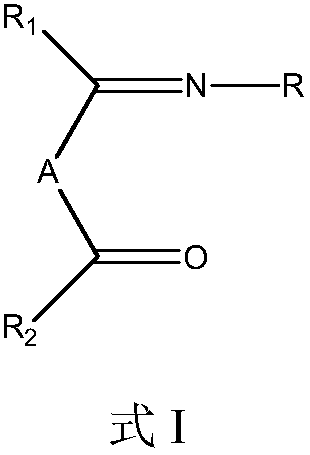
[0043] Synthesis of 4-(2,6-diisopropylphenylimino)-2-pentanone: In a three-necked flask, add 2.00 g of acetylacetone, 80 ml of isopropanol and 0.3 ml of glacial acetic acid after purging with nitrogen, Stir well at room temperature. At room temperature, a solution of 3.54 g of 2,6-diisopropylaniline in 50 ml of isopropanol was slowly added dropwise. After the addition, the mixture was stirred and reacted for 4 hours, then heated and refluxed for 16 hours. The reaction solution was concentrated under reduced pressure and separated by column chromatography to obtain 3.52 g of 4-(2,6-diisopropylphenylimino)-2-pentanone (68% yield). 1 H-NMR (δ, ppm, TMS, CDCl 3 ):7.63~7.60(1H,m,ArH),7.02~6.98(2H,m,ArH),3.35~3.31(2H,m,CH),3.24~3.20(2H,s,CH 2 ),2.10~2.07(3H,s,CH 3 ),1.38~1.32(6H,m,CH 3 ),1.25~1.21(6H,m,CH 3 ),1.03~0.98(3H,m,CH 3 ); Mass Spectrum, FD-MS: 259.
Embodiment 2
[0045] Synthesis of 4-phenylimino-2-pentanone: In a 250 ml three-necked flask, after purging with nitrogen, add 2.00 g of acetylacetone, 60 ml of absolute ethanol and 15 ml of toluene, and stir evenly at room temperature. At room temperature, 1.92 g of aniline dissolved in 40 ml of ethanol solution was slowly added dropwise, stirred and reacted for 2 hours, then heated and refluxed for 12 hours. The reaction solution was concentrated under reduced pressure and separated by column chromatography to obtain 2.03 g of 4-phenylimino-2-pentanone (yield 58%). 1 H-NMR (δ, ppm, TMS, CDCl 3 ):7.96~7.90(3H,m,ArH),7.53~7.47(2H,m,ArH),3.22~3.18(2H,s,CH 2 ),2.10~2.06(3H,s,CH 3 ),1.02~0.97(3H,s,CH 3 ); Mass Spectrum, FD-MS: 175.
Embodiment 3
[0047] Synthesis of 4-(2,6-dimethylbenzimido)-2-pentanone: In a three-necked flask, add 2.00 g of acetylacetone, 80 ml of isopropanol and 0.2 ml of glacial acetic acid after purging with nitrogen, Stir well at room temperature. At room temperature, a solution of 2.42 g of 2,6-dimethylaniline in 30 ml of isopropanol was slowly added dropwise. After the addition was completed, the mixture was stirred for 2 hours and then heated to reflux for 18 hours. The reaction solution was concentrated and separated by column chromatography to obtain 1.42 g of 4-(2,6-dimethylbenimino)-2-pentanone (yield 70%) as a light yellow liquid. 1 H-NMR (δ, ppm, TMS, CDCl 3 ):7.62~7.59(1H,m,ArH),7.02~6.98(2H,m,ArH),3.31~3.28(2H,m,CH 2 ),2.54~2.51(6H,m,CH 3 ),2.10~2.07(3H,s,CH 3 ),1.04~0.99(3H,s,CH 3 ); Mass Spectrum, FD-MS: 203.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com