Method for greatly improving chemiluminescence magnetic enzyme immunization sensitivity by depending on polystyrene nanoparticles
A polystyrene nano, chemiluminescence technology, applied in scientific instruments, material testing products, measuring devices, etc., can solve the problem of insufficient sensitivity, and achieve the effects of high sensitivity, fast detection speed and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
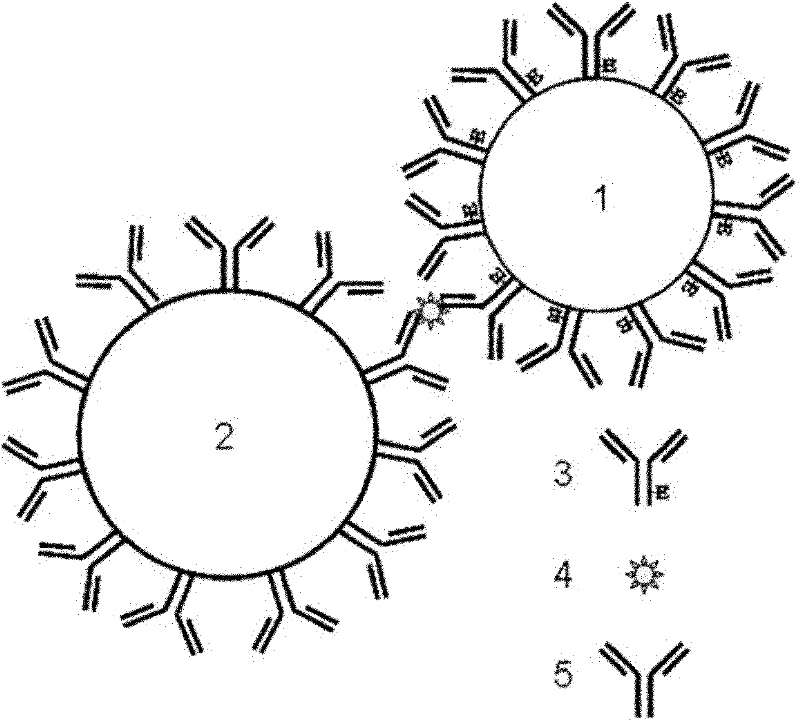
[0030] A kit for detecting cTnI, comprising sensitized nanoparticles 1, that is, polystyrene latex nanoparticle suspension covalently coupled with horseradish peroxidase-labeled mouse anti-human cTnI monoclonal antibody; sensitized magnetic particles 2 That is, the magnetic particle suspension covalently coupled with another mouse anti-human cTnI monoclonal antibody; 0.1mM chemiluminescence substrate luminol solution, 0.1mM luminescence enhancer p-iodophenol solution, and 3mM hydrogen peroxide solution. Add cTnI to the system containing the above two particles, and form a magnetic particle-antibody-antigen to be tested-enzyme-labeled antibody-polystyrene latex nanoparticle complex through the interaction of antibody-antigen-enzyme-labeled antibody, using magnetic separation technology Capture the complex, add the prepared luminol, p-iodophenol and hydrogen peroxide, and use a chemiluminescence detector to detect the luminescence intensity of the chemiluminescent substrate to c...
Embodiment 2
[0038] 1. Experimental equipment and reagents:
[0039] EnSpire multi-marker microplate detection system (PerkinElmer, USA), 96-well white plate (OptiPlate-96, PerkinElmer, USA), constant temperature water bath (HH-60, Changzhou Guohua Electric Co., Ltd.), constant temperature shaker (Taicang City Science and Education Equipment Factory), micropipette (Thermo Company), high-speed refrigerated centrifuge (TGL-16, Hunan Xiangyi Laboratory Instrument Development Co., Ltd.), 96-well plate magnetic separation rack (Tianjin Basele Chromatography Technology Development Center) . cTnI standard (BIO-RAD company), PBS-T buffer (KH 2 PO 4 3.35g / L, Na 2 HPO 4 -12H 2 O 17.9g / L, KCl 0.2g / L, NaCl 8.77g / L, Tween-200.5g / L, pH 6.96), the cTnI standard substance diluent is the PBS-T damping fluid containing 20% bovine serum, detects the cTnI The preparation of the kit is the same as in Example 1.
[0040] 2. Experimental steps:
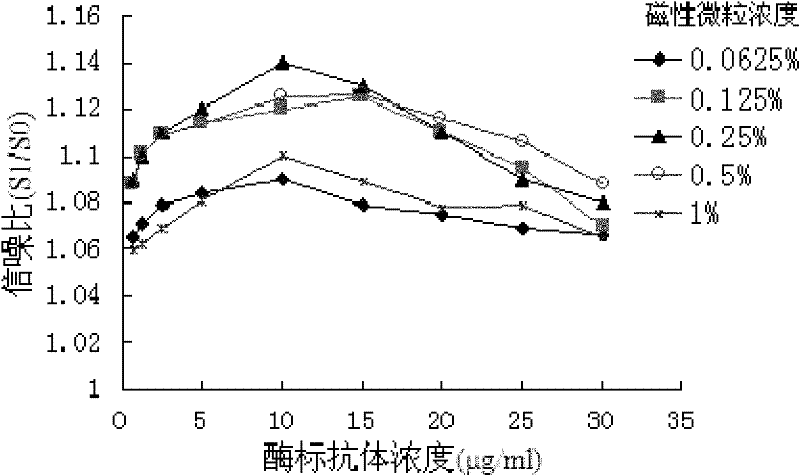
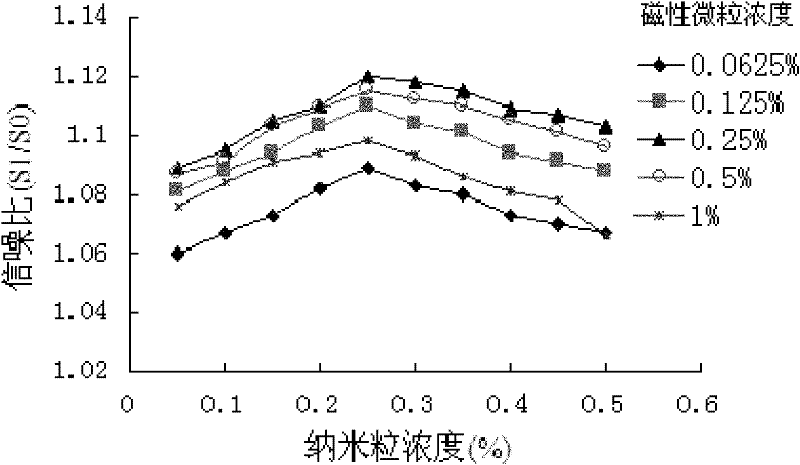
[0041] 2.1 Determination of the concentration of sensiti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com