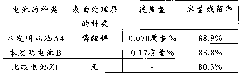Non-aqueous electrolyte secondary battery and method for producing the same
A non-aqueous electrolyte and secondary battery technology, which is applied in the direction of non-aqueous electrolyte batteries, electrolyte battery manufacturing, and non-aqueous electrolyte battery electrodes. Improved storage properties and higher capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] In Example 1, a battery was produced in the same manner as described above for implementing the invention.
[0053] The battery produced in this way is hereinafter referred to as battery A1 of the present invention.
Embodiment 2
[0055] When a phosphoric acid compound is precipitated on the surface of lithium cobaltate, samarium nitrate hexahydrate is used instead of neodymium nitrate hexahydrate, and samarium phosphate is uniformly dispersed and adhered on the surface of lithium cobaltate to obtain a positive electrode active material. In addition to using the positive electrode active material, A battery was produced in the same manner as in Example 1 above. In addition, the ratio of samarium phosphate to lithium cobaltate was 0.062% by mass in terms of samarium element, which was the same amount as the neodymium phosphate in Example 1 in terms of moles.
[0056] The battery produced in this way is hereinafter referred to as battery A2 of the present invention.
Embodiment 3
[0058] When a phosphoric acid compound is precipitated on the surface of lithium cobaltate, europium nitrate hexahydrate is used instead of neodymium nitrate hexahydrate, and europium phosphate is uniformly dispersed and adhered on the surface of lithium cobaltate to obtain a positive electrode active material. , and the same operation as in the above-mentioned Example 1 to produce a battery. In addition, the ratio of europium phosphate to lithium cobaltate was 0.063% by mass in terms of europium element, which was the same amount as the neodymium phosphate in Example 1 in terms of moles.
[0059] The battery produced in this way is hereinafter referred to as battery A3 of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| packed density | aaaaa | aaaaa |
| packed density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com