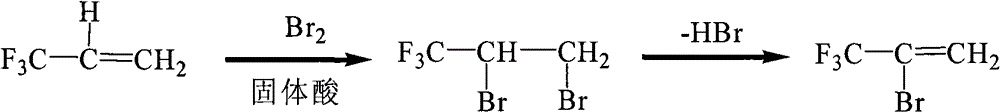
Preparation method of 2-bromine-3,3,3-trifluoropropene
A technology of trifluoropropene and liquid bromine, applied in the direction of dehydrohalogenation preparation, chemical instruments and methods, organic compound/hydride/coordination complex catalyst, etc. Recycling and reuse and other issues to achieve the effects of simplified operation, fast response, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0011] Catalyst preparation:
[0012] 1. Preparation of Mesoporous Sulfonic Acid Catalyst
[0013] Phenyltrimethoxysilane (phenyltfimethoxysilane) and tetraethoxysilane (tetraethylorthosilicate) were added to 20mL ethanol solution at a ratio of 3:7, 35mL of 0.1mol / L HCl solution was gradually added, and vigorously stirred at 60°C for 4h. After cooling to room temperature, 80 mL of ethanol and 135 mL of cyclohexane solution were added to the solution, and then 180 mL of water and 18 mL of concentrated ammonia solution were added, while stirring was maintained. Then a transparent gel appeared in the reaction vessel, and after the stirring was stopped, the obtained The solid gel was aged at room temperature for 7 days, then vacuum-dried and calcined to obtain a phenyl polysiloxane precursor. Soak the obtained precursor in tetrachloroethane solution overnight, then transfer it into a sulfonation reaction bottle, add 30% sulfur trioxide solution, the reaction is strongly exothermi...
Embodiment 1
[0018] (1) Add 1.6g of mesoporous sulfonic acid and 160g (1mol) of liquid bromine into a three-neck flask equipped with a thermometer, air guide tube and reflux condenser, heat up to 35°C under stirring, and start to introduce 91.2g (0.95mol) 3,3,3-Trifluoropropene was passed through for about 2 hours, and the reaction was continued to stir for 0.5 hours at a temperature of 35° C., cooled to room temperature, and the catalyst was filtered out to obtain a reaction liquid. The filtered catalyst can be reused.
[0019] (2) In a three-neck flask equipped with a thermometer, a constant pressure dropping funnel and an atmospheric distillation device, add 200 g of 25% aqueous sodium hydroxide solution, heat up to 75°C to 85°C under stirring, and add dropwise the obtained product in step (1). The reaction solution was distilled at the same time, and the fraction at 32-36°C was received to obtain 2-bromo-3,3,3-trifluoropropene. The yield of 2-bromo-3,3,3-trifluoropropene was 94.1%, and...
Embodiment 2
[0021] (1) Add 160g (1mol) of liquid bromine and 4.8g of montmorillonite K-10 into a three-neck flask equipped with a thermometer, an air guide tube and a reflux condenser, heat up to 20°C under stirring, and start to introduce 96g (1.0mol) ) 3,3,3-trifluoropropene, pass through for about 4 hours, continue to stir and react at a temperature of 20° C. for 0.5 hours, cool to room temperature, filter out the catalyst, and obtain a reaction liquid.
[0022] (2) The operation steps are the same as step (2) of Example 1, and the yield of the obtained 2-bromo-3,3,3-trifluoropropene is 90.6%, and the purity is 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com