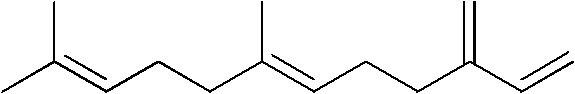
EBF ((E)-beta-Farnesene) analogues containing pyrazole-carboxamide, and preparation method and application thereof
A technology of pyrazole carboxylic acid and methyl, which is applied in the field of pyrazole cyclic carboxamide-based EBF analogs and their preparation and application, can solve the problems of easy oxidation and limited application, and achieve low cost, simple operation and direct chemical control effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, compound I1 (R 1 is methyl; R 2 is tert-butyl; R 3 is hydrogen; X is hydrogen)
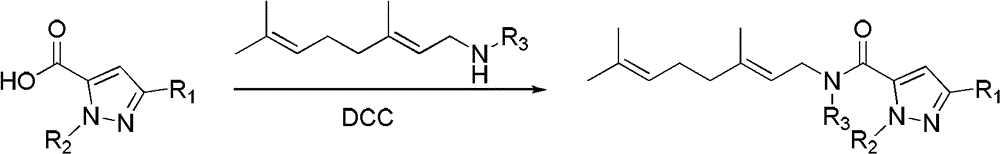
[0040] Add 0.91g (5mmol) N-tert-butyl-3-methyl-5-pyrazole acid shown in formula IIa to 50mL there-necked flask (in formula IIa, R 1 is methyl, R 2 tert-butyl), 20mL (26.5g) organic solvent dichloromethane, geranylamine compounds shown in 0.77g (5mmol) formula III (in this formula III, R 3 hydrogen, geranylamine) and 1.24g (6mmol) dicyclohexylcarbodiimide (DCC), stirring, condensation reaction at 20°C for 12h, filtering, washing the mother liquor with water (100mL×3), drying the organic phase, and concentrating , separated by column chromatography [V (petroleum ether) / V (ethyl acetate)=5:1] to obtain 1.1 g of light yellow oily liquid, which provides compound I1 (R 1 is methyl; R 2 is tert-butyl; R 3 is hydrogen; X is hydrogen), and the yield is 69.5%.
[0041] (Formula I)
[0042]
[0043] (Formula IIa)
[0044]
[0045] (Formula III)
[0046] According to th...
Embodiment 2
[0047] Embodiment 2, compound I11 (R 1 is methyl; R 2 is tert-butyl; R 3 is methyl; X is the preparation of hydrogen)
[0048] Add 0.91g (5mmol) shown in formula IIa to 50mL there-necked flask (in formula IIa, R 1 is methyl, R 2 tert-butyl) N-tert-butyl-3-methyl-5-pyrazole acid, 28mL (37.1g) organic solvent dichloromethane, 1.0g (6mmol) geranylamine compounds shown in formula III (the formula III, R 3 is methyl, N-methylgeranylamine) and 1.45g (7mmol) dicyclohexylcarbodiimide (DCC), stirred, condensation reaction was carried out at 40°C for 15h, filtered, and the mother liquor was washed with water (100mL×3), The organic phase was dried, concentrated, and separated by column chromatography [V (petroleum ether) / V (ethyl acetate)=6:1] to obtain 0.88 g of yellow oily liquid, which was the compound I11 (R 1 is methyl; R 2 is tert-butyl; R 3 is methyl; X is hydrogen), and the yield is 52.9%.
[0049] (Formula I)
[0050]
[0051] (Formula IIa)
[0052]
[0053] (...
Embodiment 3
[0055] Embodiment 3, compound I21 (R 1 is methyl; R 2 is tert-butyl; R 3 is hydrogen; X is chlorine)
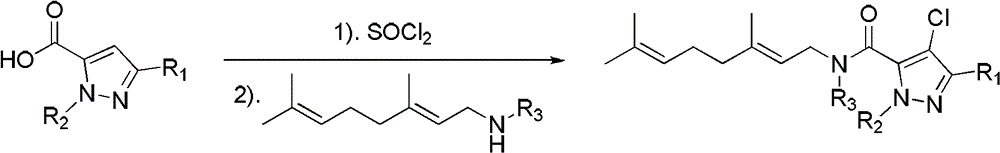
[0056] Shown in 1.27g (7mmol) formula IIb (in the formula IIb, R 1 is methyl, R 2 tert-butyl) N-tert-butyl-3-methyl-5-pyrazolecarboxylic acid was added to a 50ml three-necked flask, 11.9g (100mmol) thionyl chloride was added under ice-cooling, stirred, and refluxed at 80°C for 8 hours, Reaction finishes, and excess thionyl chloride is evaporated under reduced pressure, adds 30ml (23.7g) organic solvent anhydrous acetonitrile, stirs, adds the geranylamine compound shown in 0.77g (5mmol) formula III (in this formula III, R 3 hydrogen, geranylamine) and 12.6mmol acid-binding agent pyridine, reflux condensation reaction at 80°C for 8 hours, the reaction is completed, filtered, concentrated, and separated by column chromatography [V (petroleum ether) / V (ethyl acetate) =8:1] to obtain 1.3 g of light yellow oily liquid, which is the compound I21 (R 1 is methyl; R 2 is tert-bu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com