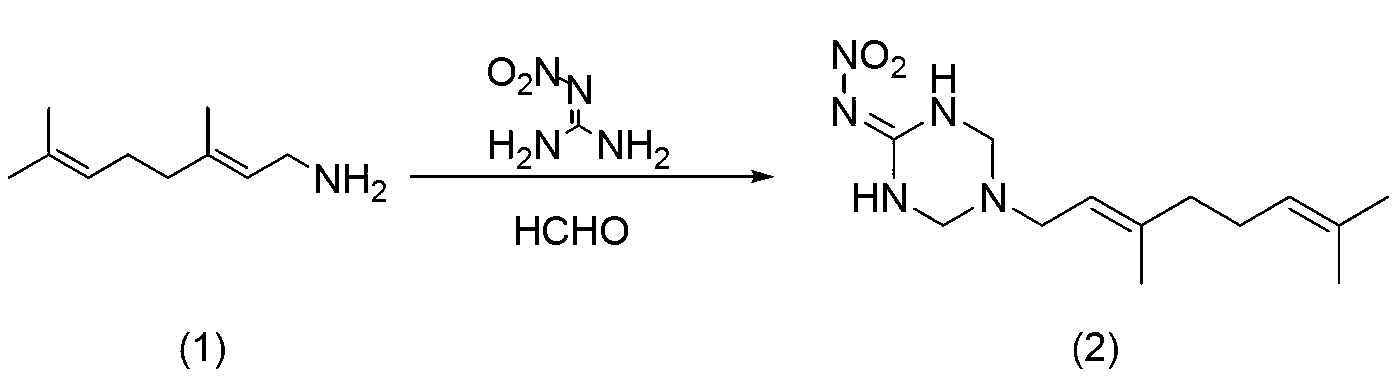
Aromatic heterocyclic triazine (trans)-delta-farnesene analogue and application thereof
A technology of aromatic heterocycles and triazines, which is applied in the field of aphid control with aromatic heterocycle triazine-β-farnesene analogues, and can solve the problem of volatile, poor stability, and limited field aphid control, etc. problem, to achieve the effect of simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: 1-(6-chloro-3-pyridylmethyl)-5-((E)-3,7-dimethyl-2,6-octadienyl)-1,3,5-hexa Preparation of hydrotriazine-2-N-nitroimine (No. 1)
[0025] Step 1: 5-((E)-3,7-Dimethyl-2,6-octadienyl)-1,3,5-hexahydrotriazine-2-N-nitroimine (intermediate 2) Preparation
[0026] In a 100ml three-necked flask, add 3.00g geranylamine, 2.00g nitroguanidine and 20mL ethanol, drop 1.58g formaldehyde solution, and react at 40°C for 3 hours. Filter, rinse the filter cake with ethanol, and dry to obtain a white solid 5-((E)-3,7-dimethyl-2,6-octadienyl)-1,3,5-hexahydrotriazine-2 -N-nitroimine (intermediate 2) 3g, yield 54%.
[0027] Step 2: 1-(6-Chloro-3-pyridylmethyl)-5-((E)-3,7-dimethyl-2,6-octadienyl)-1,3,5-hexahydro Preparation of triazine-2-N-nitroimine (No. 1)
[0028] In a 100ml three-necked flask, add 0.5g 2-chloro-5-chloromethylenepyridine, 0.64g potassium carbonate, 0.86g 5-((E)-3,7-dimethyl-2,6-octadienyl) -1,3,5-hexahydrotriazine-2-N-nitroimine and 20mL acetonitrile, refl...
Embodiment 2
[0030] Example 2: 1,3-bis(6-chloro-3-pyridylmethyl)-5-((E)-3,7-dimethyl-2,6-octadienyl)-1,3, Preparation of 5-hexahydrotriazine-2-N-nitroimine (No. 9)
[0031]In a 100ml three-necked flask, add 1g 2-chloro-5-bromomethylpyridine, 0.64g triethylamine, 0.86g 5-((E)-3,7-dimethyl-2,6-octadienyl) -1,3,5-hexahydrotriazine-2-N-nitroimine (intermediate 2) and 20 mL of benzene, reflux for 4 hours. Filtration, removal of solvent under reduced pressure, separation by silica gel column chromatography (eluent: ethyl acetate: dichloromethane V: V = 1: 1) to give white solid 1,3-bis(6-chloro-3-pyridine Methyl)-5-((E)-3,7-dimethyl-2,6-octadienyl)-1,3,5-hexahydrotriazine-2-N-nitroimine (No. 9) 0.93g, yield 57%. 1 H NMR: 1.535 (s,3H,CH 3 ),1.597(s,3H,CH 3 ),1.666(s,3H,CH 3 ),1.956-2.032(m,4H,),3.141(d, 3 J HH =6.6Hz,2H,CH 2 ),4.270(s,4H,CH 2 ),4.606(s,4H,CH 2 ),4.914-4.969(m,1H),4.997-5.037(m,1H),7.389(d,J HH =8.1Hz,2H),7.794-7.829(m,2H),8.324(d,J HH =2.4Hz, 2H).
[0032] The targe...
Embodiment 4
[0036] Embodiment 4: The insecticidal activity of the compound of the present invention to aphids
[0037] Weigh 50 mg of the target compound sample in a 20 ml weighing bottle with a ten-thousandth balance, and introduce it into a 10 mL volumetric flask to prepare a 5000 mg / L measuring solution. Then use a 1-5ml pipette gun to take 1ml of acetone and add it to a weighing bottle, add 9ml of an aqueous solution containing 0.1% Triton X-100, and mix well to obtain a 500mg / L measuring solution. Soybean leaves cultivated indoors that have not been exposed to any pesticides and insects are punched out with a 15mm diameter hole puncher, soaked in the diluted medicinal solution for 15 seconds, taken out to dry, and placed in a bioassay plate. With the back facing up, 1% agar was added to the bottom to keep it moist, and 20 soybean aphids were inserted into each well, and each was repeated 3 times. Check the results after 48 hours. The criteria for judging the death are: touch the in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com