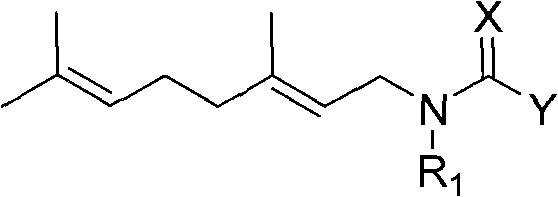
Anti-beta-farnesene analog containing guanidine or nitro-ethylene as well as preparation method and applications thereof
A technology of nitroethylene and farnesene, which is applied in the fields of botanical equipment and methods, preparation of organic compounds, preparation of amino-substituted functional groups, etc., can solve the problems of limited practical application, poor oxidation stability, etc. The effect of improved stability and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
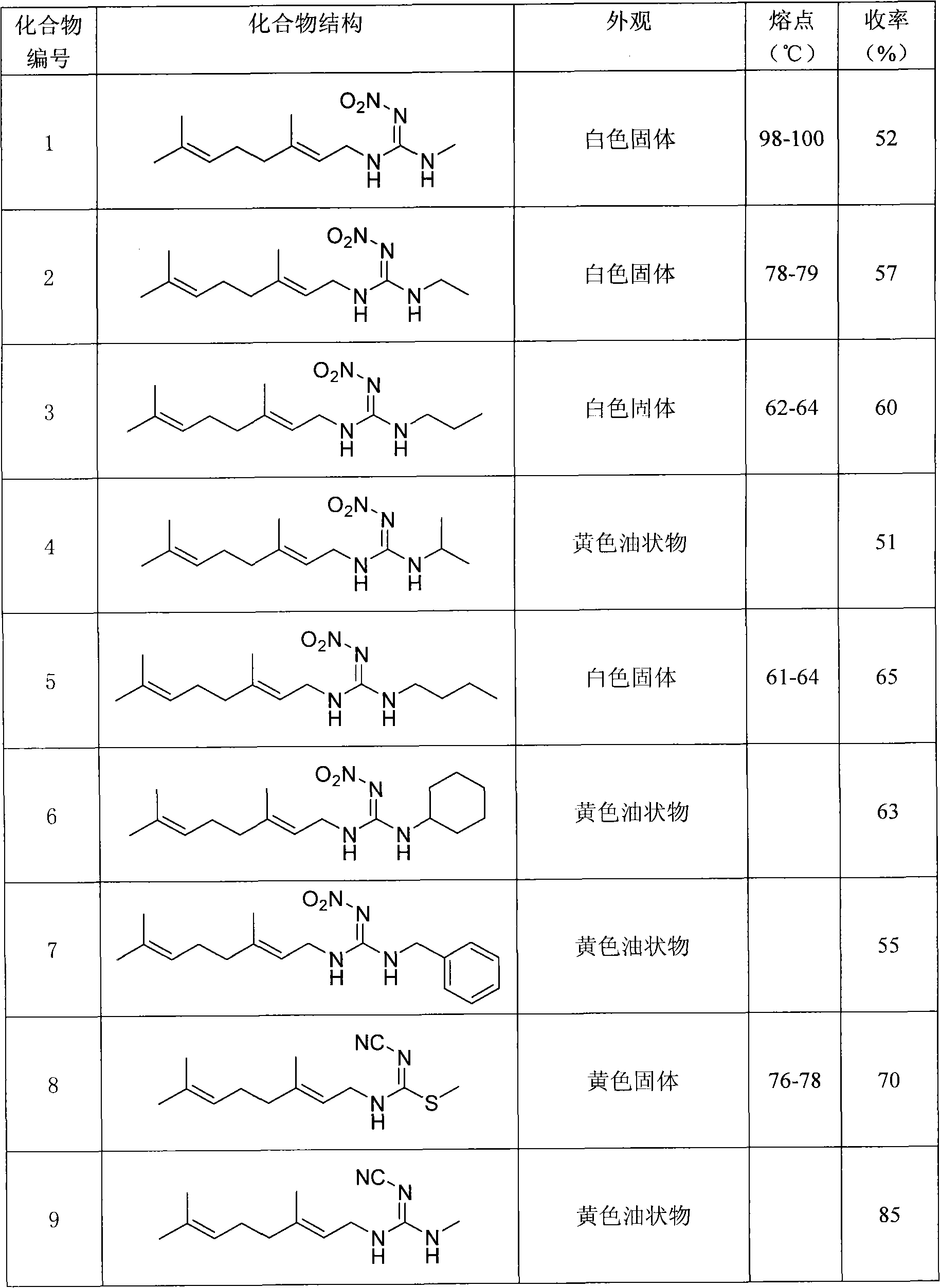
[0022] Preparation of Example 1N-((E)3,7-dimethyl-2,6-octadienyl)-N-methyl-N'-methylnitroguanidine (number 24)
[0023] Step 1 Preparation of S-methyl-N-methyl-N'-nitroisothiourea
[0024] In a 50ml three-necked flask, add 3.00g of S-methyl-N-nitrophthaloyl isothiourea, 1ml of pyridine and 20ml of dichloromethane, and dropwise add 0.95g of 37% formazan in an ice bath to 0°C The mixture of amine methanol solution and 10ml of dichloromethane was added dropwise within 30 minutes, and reacted at 25°C for 3 hours. Filtration, the filtrate was decompressed to remove the solvent to obtain a yellow oil, which was separated by silica gel column chromatography (eluent: ethyl acetate:petroleum ether V:V=1:5) to obtain a white solid S-methyl-N-methyl -N'-Nitroisothiourea 1.3g, yield 85%, melting point 143°C-145°C.
[0025] Preparation of Step 2N-((E)3,7-dimethyl-2,6-octadienyl)-N-methyl-N'-methylnitroguanidine
[0026] In a 50ml three-necked flask, add 0.5g of S-methyl-N-methyl-N′-nitr...
Embodiment 2
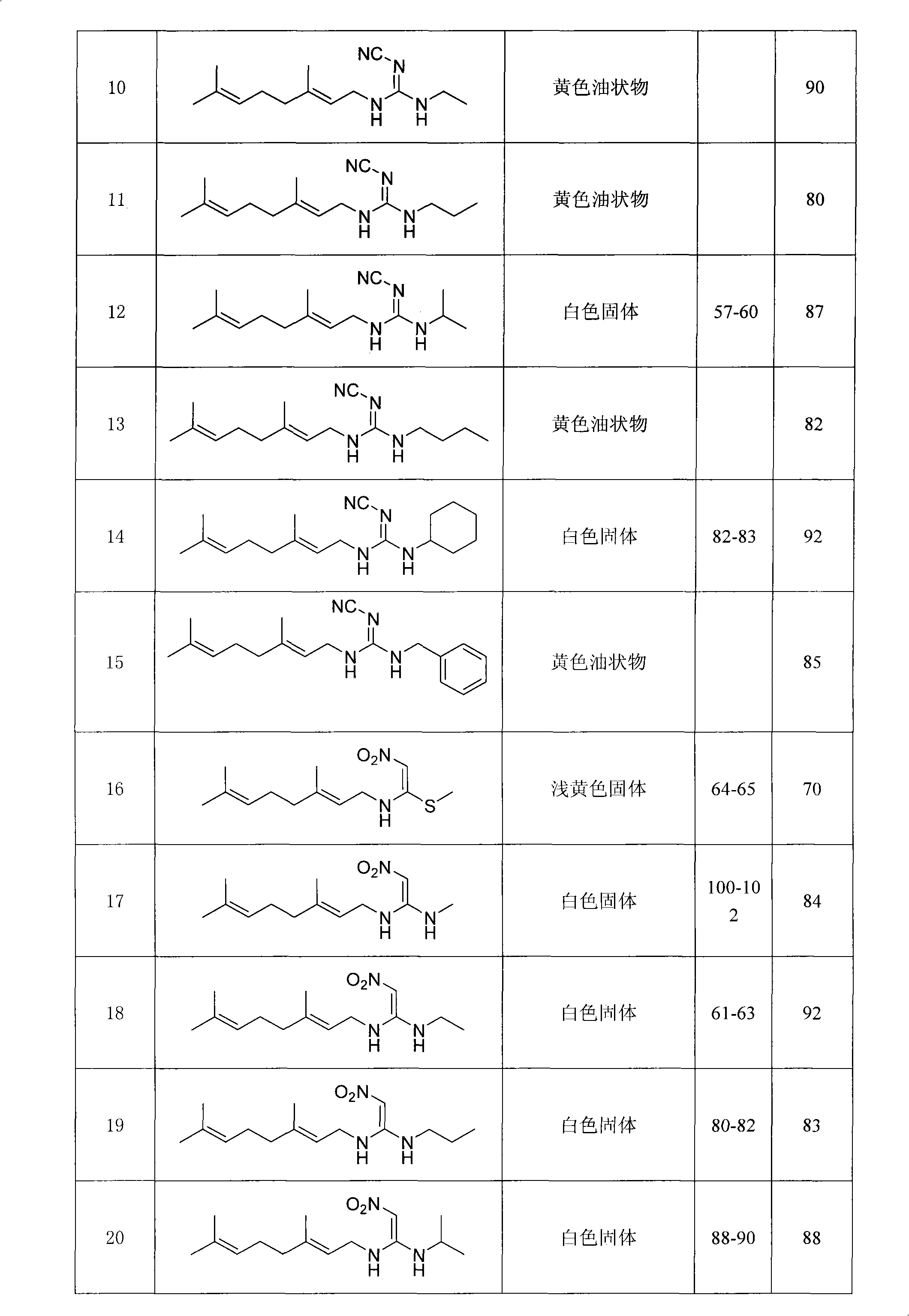
[0028] Preparation of Example 2N-((E)3,7-dimethyl-2,6-octadienyl)-N-methyl-N'-methylcyanoguanidine (numbering 30)
[0029] Step 1 Preparation of N-((E)3,7-dimethyl-2,6-octadienyl)-N-methyl-N'-cyano-S-methylisothiourea
[0030] In a 50ml three-necked flask, add 2.00g of dimethyldithiocyanoimine, 30ml of acetonitrile and 2.29g of N-methylgeranylamine, reflux at 80-81°C for 5h, remove the solvent under reduced pressure, and use a silica gel column layer Analysis and separation (eluent: ethyl acetate:petroleum ether V:V=1:4) gave yellow oil N-((E)3,7-dimethyl-2,6-octadienyl)- N-methyl-N'-cyano-S-methylisothiourea 1.93g, yield 56%. 1HNMR: 1.58-1.70(s, 9H, CH3-C=C), 2.04-2.13(m, 4H, CH2-CH2-C=C), 2.79(s, 3H, S-CH3), 3.13(s, 3H , N-CH3), 4.20-4.22 (d, 2H, CH3-N-CH2), 5.03-5.14 (t, 2H, CH=C).
[0031] Preparation of step 2N-((E)3,7-dimethyl-2,6-octadienyl)-N-methyl-N'-methylcyanoguanidine
[0032]In a 50ml three-necked flask, add 1.00g of N-((E)3,7-dimethyl-2,6-octadienyl)-N-methy...
Embodiment 3
[0034] Example 31- Preparation of (N-((E)3,7-dimethyl-2,6-octadienyl))amino-1-methylamino-2-nitroethylene (No. 17)
[0035] Step 11 - Preparation of (N-((E)3,7-dimethyl-2,6-octadienyl))amino-1-methylthio-2-nitroethylene
[0036] In a 50ml three-necked flask, add 2.00g of 1,1-dimethylthio-2-nitroethylene, 30ml of acetonitrile and 1.85g of geranylamine, heat to 80-81°C for 5h under reflux, remove the solvent under reduced pressure, and use silica gel Column chromatography (eluent: ethyl acetate:petroleum ether V:V=1:6) gave yellow oil 1-(N-((E)3,7-dimethyl-2,6-octyl Dienyl))amino-1-methylthio-2-nitroethylene 2.29 g, yield 70%. 1HNMR: 1.60-1.70(s, 9H, CH3-C=C), 2.02-2.17(m, 4H, CH2-CH2-C=C), 2.45(s, 1H, S-CH3), 4.00-4.04(t , 2H, CH2-C=C), 5.04-5.29 (t, 2H, CH=C), 6.58 (s, 1H, CH=C-NO2), 10.39 (s, 1H, NH).
[0037] Step 21 - Preparation of (N-((E)3,7-dimethyl-2,6-octadienyl))amino-1-methylamino-2-nitroethene
[0038] In a 50ml three-necked flask, add 1.00g of 1-(N-((E)3,7-dime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com