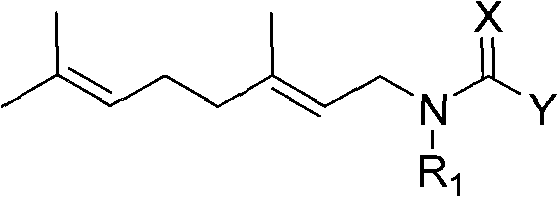
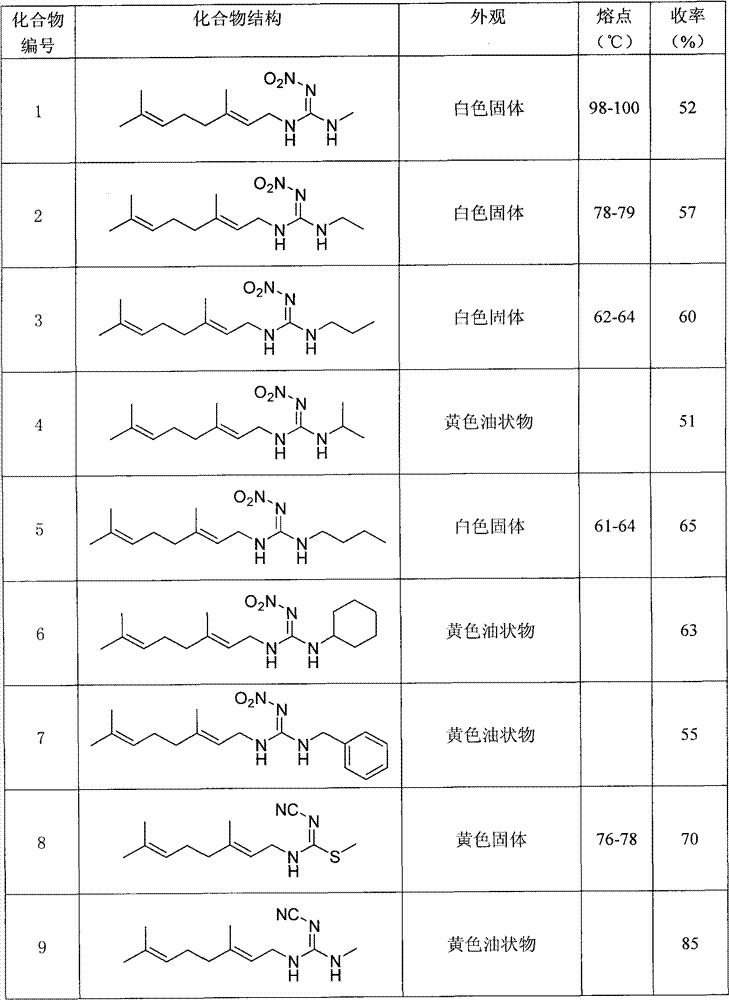
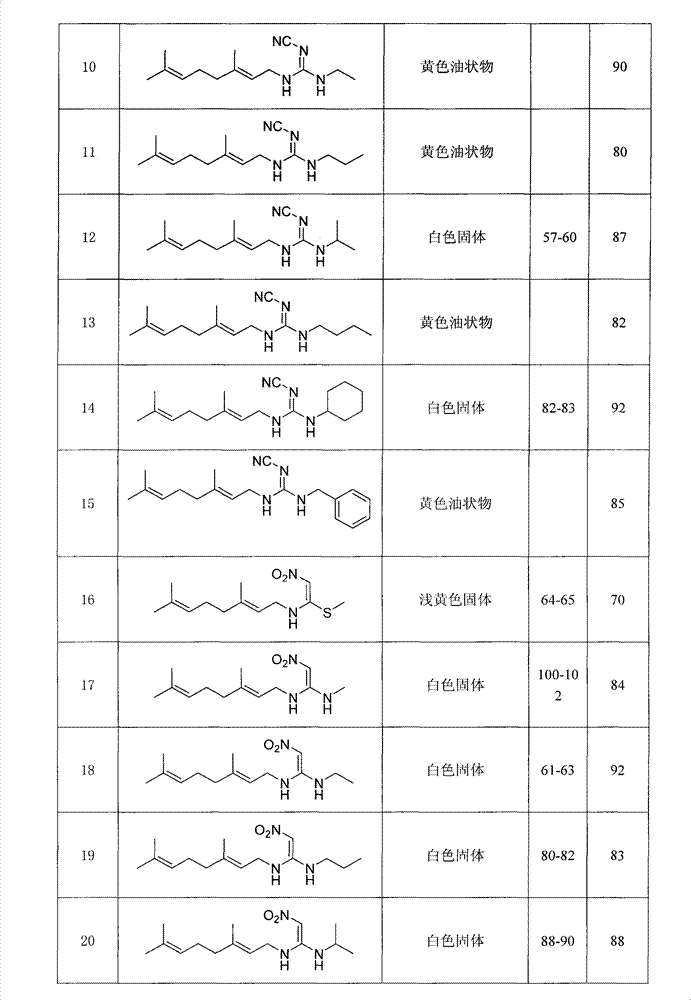
Anti-beta-farnesene analog containing guanidine or nitro-ethylene as well as preparation method and applications thereof
A technology of nitroethylene and farnesene, applied in botany equipment and methods, preparation of organic compounds, preparation of functional groups substituted with amino groups, etc., can solve the problems of limited practical application, poor oxidation stability, etc., and achieve volatility reduction, Improved stability and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of Example 1N-((E)3,7-dimethyl-2,6-octadienyl)-N-methyl-N'-methylnitroguanidine (number 24)
[0023] Step 1 Preparation of S-methyl-N-methyl-N'-nitroisothiourea
[0024] In a 50ml three-necked flask, add 3.00g of S-methyl-N-nitrophthaloyl isothiourea, 1ml of pyridine and 20ml of dichloromethane, and dropwise add 0.95g of 37% formazan in an ice bath to 0°C The mixture of amine methanol solution and 10ml of dichloromethane was added dropwise within 30 minutes, and reacted at 25°C for 3 hours. Filtration, the filtrate was decompressed to remove the solvent to obtain a yellow oil, which was separated by silica gel column chromatography (eluent: ethyl acetate:petroleum ether V:V=1:5) to obtain a white solid S-methyl-N-methyl -N'-Nitroisothiourea 1.3g, yield 85%, melting point 143°C-145°C.
[0025] Preparation of Step 2N-((E)3,7-dimethyl-2,6-octadienyl)-N-methyl-N'-methylnitroguanidine
[0026] In a 50ml three-necked flask, add 0.5g of S-methyl-N-methyl-N′-nitr...
Embodiment 2
[0028] Preparation of Example 2N-((E)3,7-dimethyl-2,6-octadienyl)-N-methyl-N'-methylcyanoguanidine (numbering 30)
[0029] Step 1 Preparation of N-((E)3,7-dimethyl-2,6-octadienyl)-N-methyl-N'-cyano-S-methylisothiourea
[0030] In a 50ml three-necked flask, add 2.00g of dimethyldithiocyanoimine, 30ml of acetonitrile and 2.29g of N-methylgeranylamine, reflux at 80-81°C for 5h, remove the solvent under reduced pressure, and use a silica gel column layer Analysis and separation (eluent: ethyl acetate:petroleum ether V:V=1:4) gave yellow oil N-((E)3,7-dimethyl-2,6-octadienyl)- N-methyl-N'-cyano-S-methylisothiourea 1.93g, yield 56%. 1HNMR: 1.58-1.70(s, 9H, CH3-C=C), 2.04-2.13(m, 4H, CH2-CH2-C=C), 2.79(s, 3H, S-CH3), 3.13(s, 3H , N-CH3), 4.20-4.22 (d, 2H, CH3-N-CH2), 5.03-5.14 (t, 2H, CH=C).
[0031] Preparation of step 2N-((E)3,7-dimethyl-2,6-octadienyl)-N-methyl-N'-methylcyanoguanidine
[0032]In a 50ml three-necked flask, add 1.00g of N-((E)3,7-dimethyl-2,6-octadienyl)-N-methy...
Embodiment 3
[0034] Example 31- Preparation of (N-((E)3,7-dimethyl-2,6-octadienyl))amino-1-methylamino-2-nitroethylene (No. 17)
[0035] Step 11 - Preparation of (N-((E)3,7-dimethyl-2,6-octadienyl))amino-1-methylthio-2-nitroethylene
[0036] In a 50ml three-necked flask, add 2.00g of 1,1-dimethylthio-2-nitroethylene, 30ml of acetonitrile and 1.85g of geranylamine, heat to 80-81°C for 5h under reflux, remove the solvent under reduced pressure, and use silica gel Column chromatography (eluent: ethyl acetate:petroleum ether V:V=1:6) gave yellow oil 1-(N-((E)3,7-dimethyl-2,6-octyl Dienyl))amino-1-methylthio-2-nitroethylene 2.29 g, yield 70%. 1HNMR: 1.60-1.70(s, 9H, CH3-C=C), 2.02-2.17(m, 4H, CH2-CH2-C=C), 2.45(s, 1H, S-CH3), 4.00-4.04(t , 2H, CH2-C=C), 5.04-5.29 (t, 2H, CH=C), 6.58 (s, 1H, CH=C-NO2), 10.39 (s, 1H, NH).
[0037] Step 21 - Preparation of (N-((E)3,7-dimethyl-2,6-octadienyl))amino-1-methylamino-2-nitroethene
[0038] In a 50ml three-necked flask, add 1.00g of 1-(N-((E)3,7-dime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com