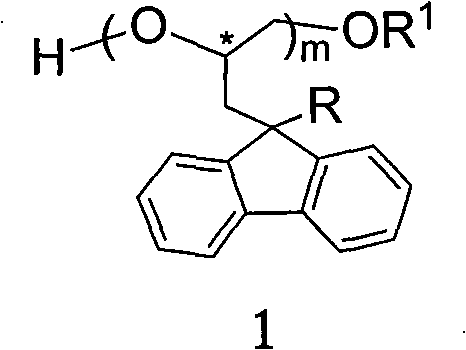
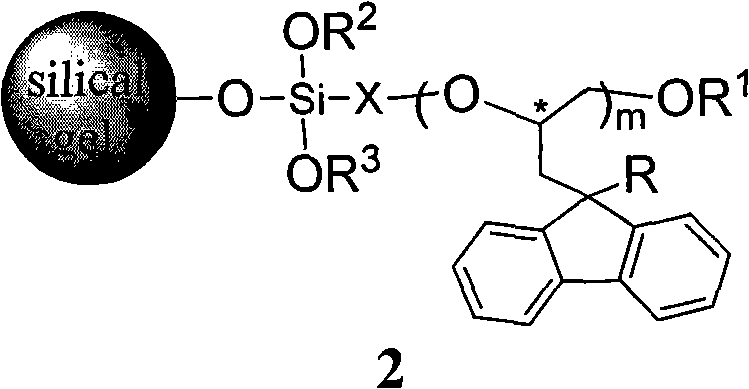
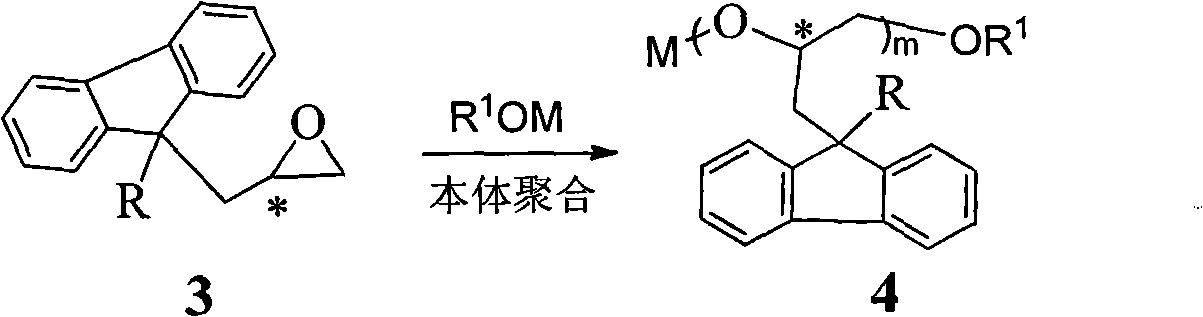
Silica gel bonded with single-chiral spiral polyether, preparation method thereof, and application thereof used as chiral immobile phase of high-performance liquid chromatographic column
A bonding and helical technology, which is applied in the field of silica gel bonded with monochiral helical polyether and its preparation and use as a chiral stationary phase in high performance liquid chromatography, which can solve problems such as application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0056] Example 1: Activation of silica gel
[0057] Put 60g of spherical silica gel into a 2000mL three-necked bottle, add 1500mL of 10% hydrochloric acid, and heat to reflux for 10h. Filter after cooling. The filter cake was washed with distilled water until the washed water was neutral, and then washed several times with acetone. After the acetone on the surface was volatilized, the filter cake was dried in an oven at 160°C for 8 hours, and then stored in a desiccator to cool for later use.
example 2
[0058] Example 2: Preparation of silica gel bonded with monochiral helical polyether having poly[(S)-3-(9-methylfluoren-9-yl)-1,2-propylene oxide] skeleton structure. method one.
[0059] Add 8 mmoles of (S)-3-(9-methylfluoren-9-yl)-1,2-propylene oxide and 0.020 g of KOH into a reaction flask equipped with a magnetic stirring bar. The reaction bottle was vacuumized and filled with high-purity argon, and the operation of pumping and filling with argon was repeated twice. Place the reaction bottle in an oil bath at 100-110°C, and react under magnetic stirring. When the reactant was solidified and the stirring bar could not be stirred, the temperature of the oil bath was lowered to 80°C, and 3 mL of anhydrous toluene was added with a syringe. After the solid block was completely dissolved, 0.4 mmol (0.095 g) of 3-glycidylpropyltrimethoxysilane was added, and the mixture was stirred and reacted in an oil bath at 80° C. for 4 hours. Then, the reaction solution was taken out with...
example 3
[0060] Example 3: Preparation of silica gel bonded with monochiral helical polyether having poly[(S)-3-(9-ethylfluoren-9-yl)-1,2-propylene oxide] skeleton structure. Method Two.
[0061] Add 8 mmoles of (S)-3-(9-ethylfluoren-9-yl)-1,2-propylene oxide into a reaction flask equipped with a magnetic stirring bar. The reaction bottle was vacuumized and filled with high-purity argon, and the operation of pumping and filling with argon was repeated twice. Inject 3 mL of anhydrous toluene and 0.2 mmol of potassium tert-butoxide in THF with a syringe. The reaction bottle was placed in an oil bath at 100-110° C., and reacted for 12 hours under magnetic stirring. The temperature of the oil bath was lowered to 80° C., 0.4 mmol (0.095 g) of 3-glycidylpropyltrimethoxysilane was added, and the mixture was stirred and reacted in an oil bath of 80° C. for 4 hours. Then, the reaction solution was taken out with a syringe, quickly injected into a three-necked bottle containing 60 mL of anhyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com