Method for manufacturing cobalt nickel lithium manganate oxide as gradient anode active material of lithium ion battery
A cathode active material, a technology of lithium cobalt nickel manganese oxide, applied in the field of cathode material preparation of lithium ion batteries, can solve the problems to be improved, low first cycle efficiency, etc., so as to improve the charge-discharge capacity and cycle efficiency, and reduce the degree of cation mixing. , the effect of good electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
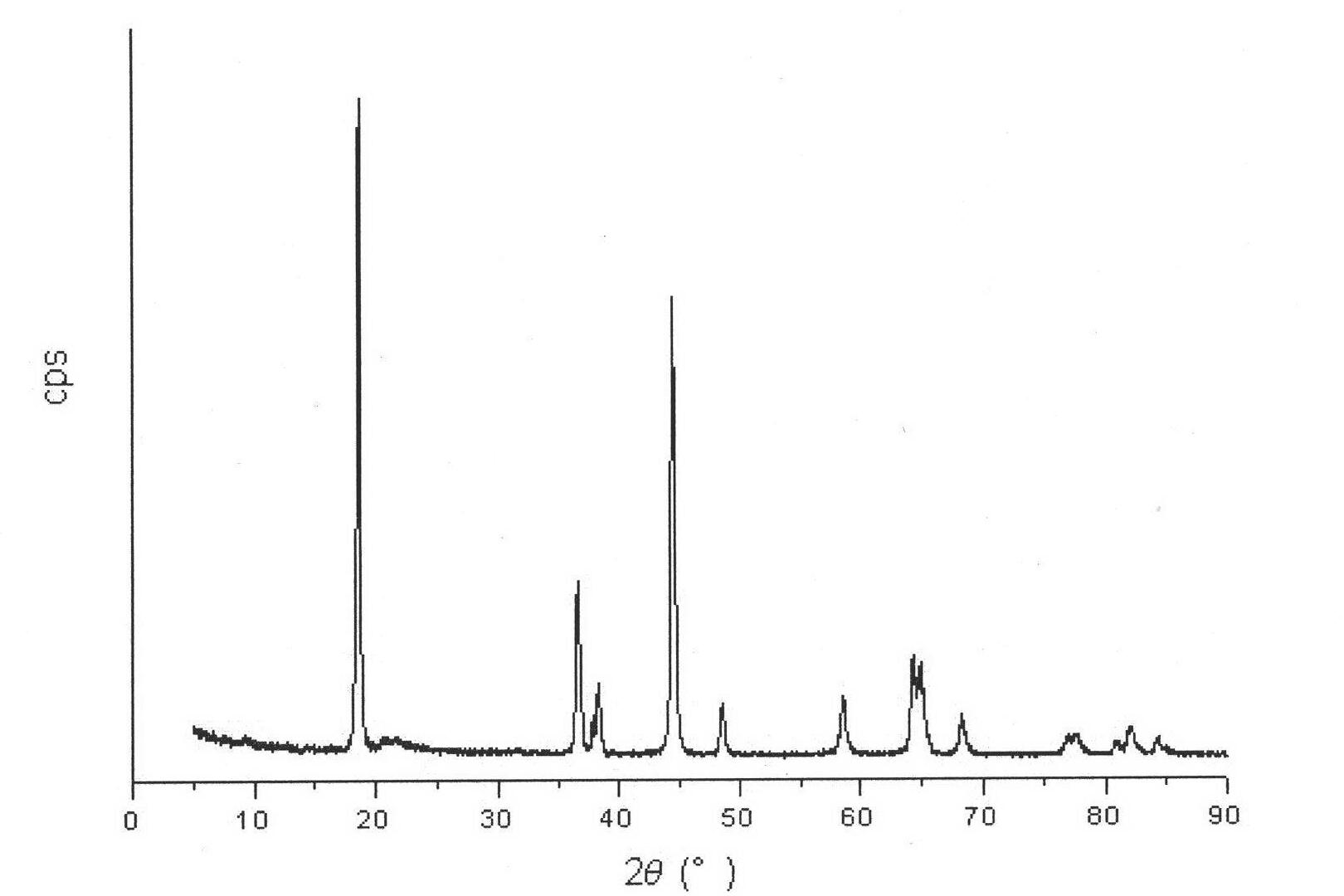
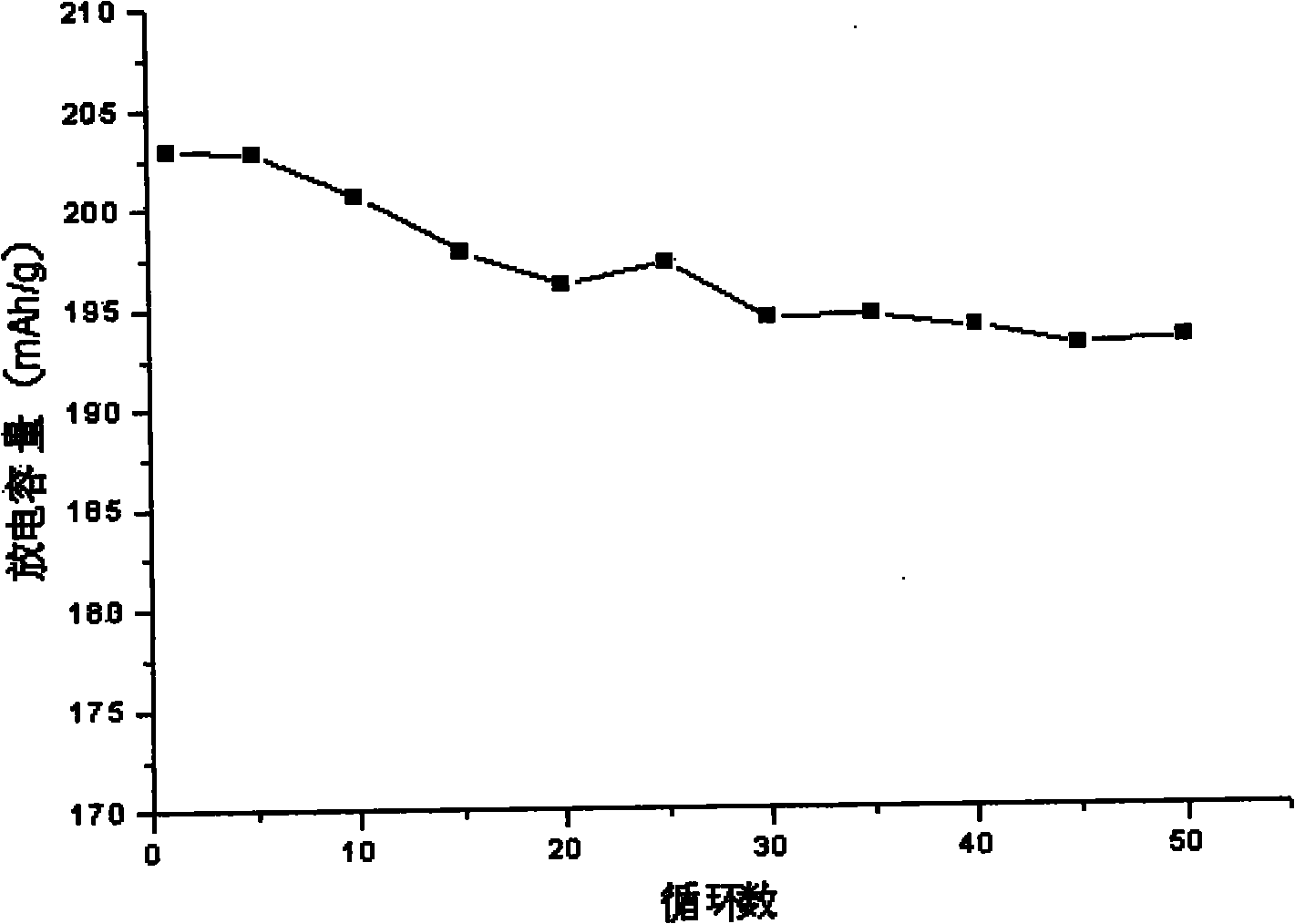
Image
Examples
Embodiment 1
[0024] A: Under the condition of constant temperature and mechanical stirring at 40°C, 6 mL of NH with a concentration of 6 mol / L 3 ·H 2 O solution and 6mL 1mol / L NaOH solution were mixed as the base liquid, and 0.2mol / L Co(NO 3 ) 2 Solution 3mL and 1mol / L Mn(NO 3 ) 2 Mix 8mL of the solution evenly, drop it into the reaction bottle through a dropper, and add 22mL of 1mol / L NaOH solution dropwise at the same time, react for 20min and then filter under reduced pressure to obtain a light brown precipitate, which is immersed in 6mol / L NH 3 ·H 2 O solution, ultrasonically disperse at room temperature for 30 min.
[0025] B: Add 1mol / L Co(NO 3 ) 2 Solution 1mL and 1mol / L Ni(NO 3 ) 2 Mix 6mL of the solution evenly, drop it into the reaction system through a dropper, and add 14mL of 1mol / L NaOH solution dropwise at the same time, react for 20min, and filter under reduced pressure to obtain a brown precipitate. Submerge the precipitate in 6mol / L NH 3 ·H 2 O solution, ultras...
Embodiment 2
[0031] A: Under the condition of constant temperature and mechanical stirring at 45°C, 7.5 mL of NH with a concentration of 6 mol / L 3 ·H 2 O solution and 7.5mL 1mol / L NaOH solution were mixed as the base liquid, and 0.3mol / L Co(NO 3 ) 2 Solution 1mL and 1mol / L Mn(NO 3 ) 2 Mix 12mL of the solution evenly, add dropwise to the reaction bottle through a dropper, and add 26mL of 1mol / L NaOH solution dropwise at the same time, react for 20min and then filter under reduced pressure to obtain a light brown precipitate, which is immersed in 6mol / L NH 3 ·H 2 O solution, ultrasonically disperse at room temperature for 30 min.
[0032] B: 0.6mol / L Co(NO 3 ) 2 Solution 1.5mL and 1mol / L Ni(NO 3 ) 2 Mix 4.6mL of the solution evenly, drop it into the reaction bottle through a dropper, and add 12mL of 1mol / L NaOH solution dropwise at the same time, react for 20min, and filter under reduced pressure to obtain a brown precipitate. Submerge the precipitate in 6mol / L NH 3 ·H 2 O soluti...
Embodiment 3
[0037] A: Under the condition of constant temperature and mechanical stirring at 45°C, 7 mL of NH with a concentration of 5 mol / L 3 ·H 2 O solution and 7mL 1mol / L NaOH solution were mixed as the base liquid, and 0.3mol / L Co(NO 3 ) 2 Solution 2mL and 1mol / L Mn(NO 3 ) 2 Mix 12mL of the solution evenly, drop it into the reaction bottle through a dropper, and add 28mL of 1mol / L NaOH solution dropwise with another dropper, react for 20min and filter under reduced pressure to obtain a light brown precipitate, which is immersed in 5mol / L NH 3 ·H 2 O solution, ultrasonically disperse at room temperature for 30 min.
[0038] B: 1mol / L Co(NO 3 ) 2 Solution 0.9mL and 1mol / L Ni(NO 3 ) 2Mix 4mL of the solution evenly, add dropwise through a dropper, and simultaneously add 10mL of 1mol / L NaOH solution dropwise, react for 20min, and filter under reduced pressure to obtain a brown precipitate. Submerge the precipitate in 5mol / L NH 3 ·H 2 O solution, ultrasonically disperse at roo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com