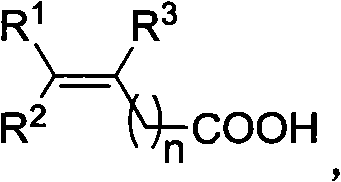
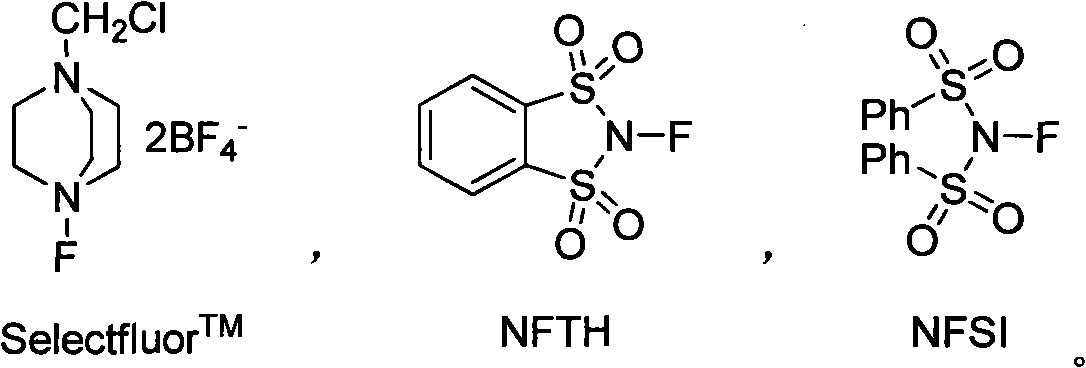
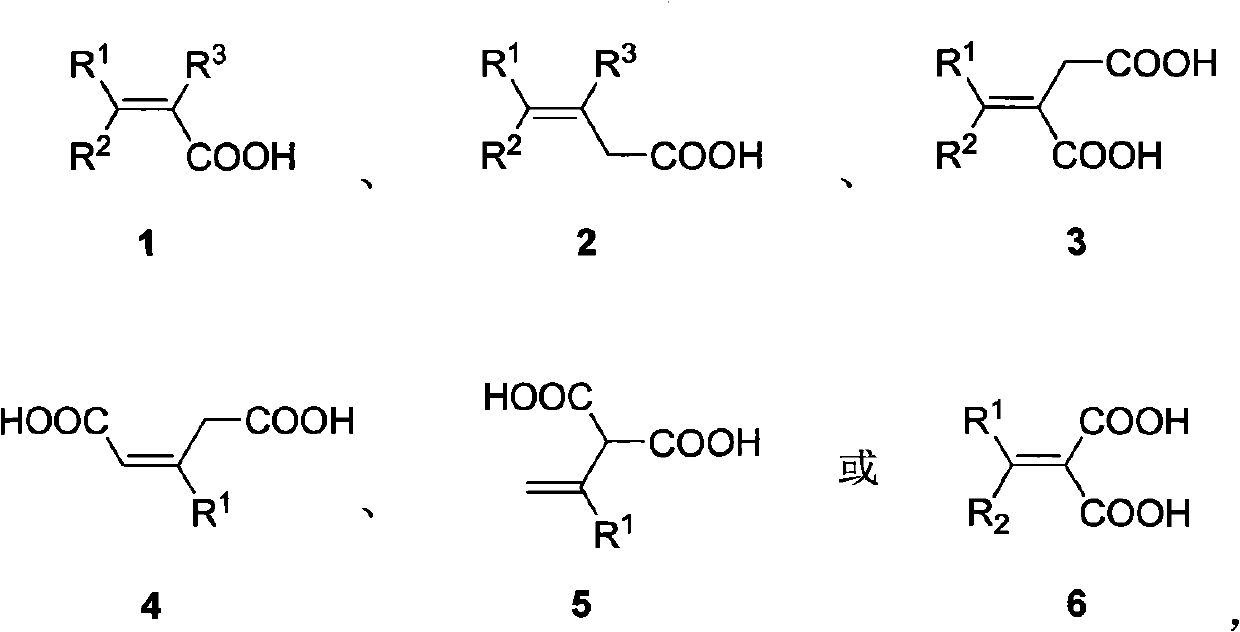
Fluorination method of vinyl carboxylic acid
A technology of alkenyl carboxylic acid and fluorination, applied in chemical instruments and methods, organic chemistry, preparation of organic compounds, etc., can solve the problem of unsaturated carboxylic acid that no one has reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079]
[0080] In a reaction tube equipped with a built-in reflux condenser, add 3,3-diphenylacrylic acid (112mg, 0.5mmol), Selectfluor (213mg, 0.6mmol), nickel acetate dihydrate (124mg, 0.5mmol), 2mL of tetrachloromethane carbonized carbon, 3 mL of water, 0.5 mL of trifluoroacetic acid, stirred, and heated to reflux for 40 hours. After cooling to room temperature, it was first neutralized to weak alkalinity with saturated aqueous sodium bicarbonate solution, and then extracted with dichloromethane. The organic layer was concentrated through a column to obtain 79 mg of a colorless liquid with a yield of 80%.
Embodiment 2
[0082]
[0083] Add 3,3-diphenylacrylic acid (112mg, 0.5mmol), Selectfluor (532mg, 1.5mmol), 5mL water, and 1mL trifluoroacetic acid in sequence in a 20mL reaction tube equipped with a built-in reflux condenser, stir, and heat to reflux 60 hours. After cooling to room temperature, it was first neutralized to weak alkalinity with saturated aqueous sodium bicarbonate solution, and then extracted with dichloromethane. The organic layer was concentrated through a column to obtain 104 mg of a colorless liquid with a yield of 89%.
Embodiment 3
[0085]
[0086] Add 3-(4-nitrophenyl)acrylic acid (104mg, 0.5mmol), Selectfluor (532mg, 1.5mmol), 5mL water, 1mL trifluoroacetic acid in turn in a 20mL reaction tube equipped with a built-in reflux condenser, and stir , heated to reflux for 60 hours. After cooling to room temperature, it was first neutralized to weak alkalinity with saturated aqueous sodium bicarbonate solution, and then extracted with dichloromethane. The organic layer was concentrated through a column to obtain 53 mg of a colorless liquid with a yield of 43%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com