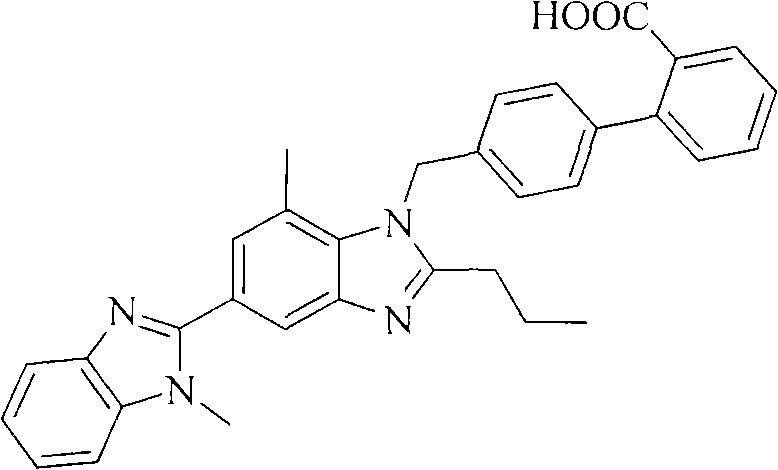
Preparation method of telmisartan impurity B
A technology for telmisartan and impurities, applied in the field of preparation of telmisartan impurity B, can solve the problems such as no public information reporting impurity B synthesis method, no sales, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
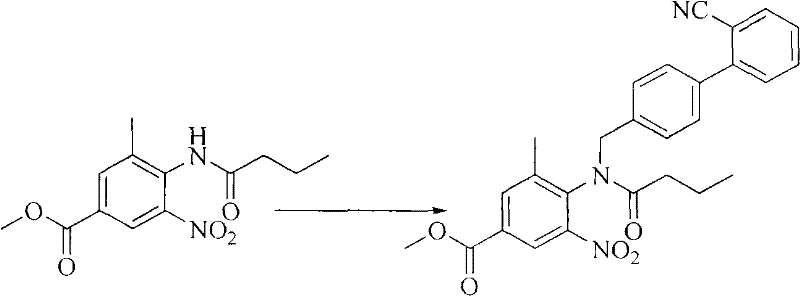
[0043] Step 1: Preparation of 4-[N-(2'-cyanobiphenyl-4-methylene)butyramide]-3-methyl-5-nitro-benzoic acid methyl ester
[0044] At room temperature, add 14.0 g of 4-butyrylamino-3-methyl-5-nitro-benzoic acid methyl ester, 13.8 g of potassium carbonate and 70 mL of dimethylformamide solvent into a 250 mL four-necked flask, and stir for 0.5 h. Then add 14.00 g of 4'-bromomethyl-biphenyl-2-carbonitrile in one go, continue to stir at room temperature for 1 h, and monitor the reaction by thin-layer chromatography (dichloromethane:methanol=20:1), the results show that the raw materials are completely consumed , generating a strong UV spot with a slightly smaller polarity.
[0045] The solvent was distilled off under reduced pressure (60°C), and a large amount of white solid was precipitated. Add 70mL of water, stir for 0.5h, filter, wash the filter cake twice with water (about 30mL each time), and dry to obtain 23.5g of white product.
[0046] The preparation of the second step 1-...
Embodiment 2
[0065] Step 1: Preparation of 4-[N-(2'-cyanobiphenyl-4-methylene)butyramide]-3-methyl-5-nitro-benzoic acid methyl ester
[0066] At room temperature, add 14.0 g of 4-butyrylamino-3-methyl-5-nitro-benzoic acid methyl ester, 16.8 g of sodium bicarbonate and 80 mL of tetrahydrofuran solvent into a 250 mL four-neck flask, stir for 1 hour, and then add 14.0 g (0.052 mol) of 4'-bromomethyl-biphenyl-2-carbonitrile was stirred at room temperature for 1 h.
[0067] The solvent was distilled off under reduced pressure (60°C), and a large amount of white solid was precipitated. Add 70mL of water, stir for 0.5h, filter, wash the filter cake twice with water (30mL×2), and dry to obtain 22.5g of white product.
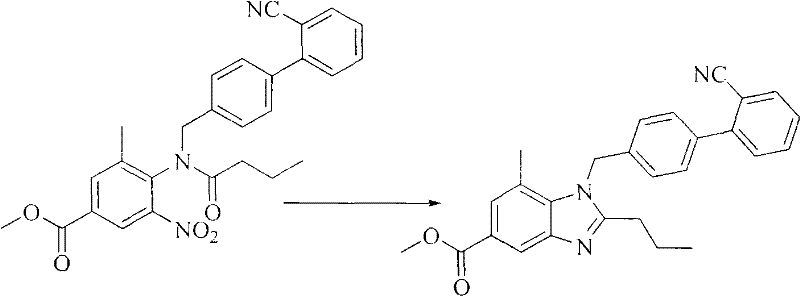
[0068] Preparation of the second step 1-(2'-cyanobiphenyl-4-methylene)-7-methyl-2-n-propyl-1H-benzimidazole-5-formic acid methyl ester
[0069] At room temperature, add 4-[N-(4'-cyanobiphenyl-2-methylene)butyrylamide]-3-methyl-5-nitro-benzoic acid methyl ester 23.6 g, 120mL of gla...
Embodiment 3
[0086] Example 3 Preparation of 1-(2'-cyanobiphenyl-4-methylene)-7-methyl-2-n-propyl-1H-benzimidazole-5-methyl carboxylate
[0087] At room temperature, add 4-[N-(4'-cyanobiphenyl-2-methylene)butyrylamide]-3-methyl-5-nitro-benzoic acid methyl ester 23.6 g, 120mL of glacial acetic acid solvent and 5.6g of aluminum powder, stirred, and slowly raised the temperature to 80°C to reduce the ring closure for 1h.
[0088] Cool to about 80°C, filter hot, rinse the aluminum sludge with 100mL dichloromethane (dichloromethane), extract the dichloromethane filtrate for later use, add 250mL water after the acetic acid filtrate spins dry, extract 3 times with dichloromethane (100mL×3 ), combined organic phases, washed once with 50mL of 10% aqueous sodium bicarbonate solution, washed once with 50mL of water, dried over anhydrous sodium sulfate, filtered, and evaporated the solvent to obtain 21.6g of light yellow oil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com