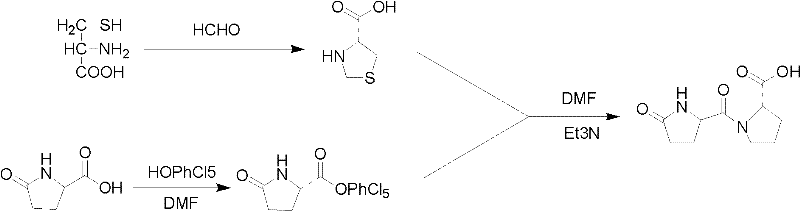
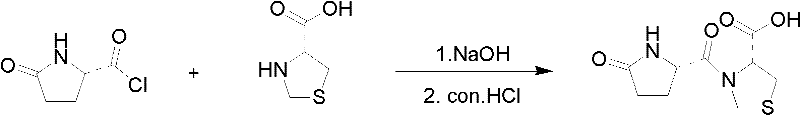Method for synthesizing pidotimod
A technology of pidotimod and a synthesis method, applied in the field of preparation of pharmaceutical compounds, can solve the problems of low yield and high production cost in the synthesis process, and achieve the effects of low price, low cost and reduced decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, preparation method of the present invention
[0030] Step 1, weigh 100g of L-thiazolidine-4-carboxylic acid and add it into 400mL of acetone, then add 114g of potassium carbonate and stir for 0.5h.
[0031] Step 2, dissolve 122g of L-pyroglutamyl chloride in 300mL of acetone, then dropwise add it to the solution obtained in step a and stir for 2h.
[0032] Step 3, filter, dissolve the filter cake in 500mL of water, ice-bath to 0-10 degrees, acidify the above solution with concentrated hydrochloric acid to PH = 2, a white precipitate is formed, continue to stir for 1 hour.
[0033] Step 4, filtering and drying. The crude product of pidotimod was obtained.
[0034] Step 5, the crude product of pidotimod is recrystallized with water, filtered, dried, and weighed to obtain 165g of refined pidotimod with a yield of 90%
Embodiment 2
[0035] Embodiment 2. preparation method of the present invention
[0036] Step 1, weigh 100g of L-thiazolidine-4-carboxylic acid and add it into 500mL of acetone, then add 96g of sodium carbonate and stir for 0.5h.
[0037] Step 2, dissolve 166g of L-pyroglutamyl chloride in 400mL of acetone, then dropwise add it to the solution obtained in step a and stir for 2h.
[0038] Step 3, filter, dissolve the filter cake in 500mL of water, ice-bath to 0-10 degrees, acidify the above solution with concentrated hydrochloric acid to PH = 2, a white precipitate is formed, continue to stir for 1 hour.
[0039] Step 4, filtering and drying. The crude product of pidotimod was obtained.
[0040] In step 5, the crude product of pidotimod was recrystallized with water, filtered, dried, and weighed to obtain 163g of refined pidotimod with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com