Novel method for synthesizing lacosamide
A technology for synthesizing lacosamide and a new method, which is applied in the field of improved preparation of 2-acetamido-N-benzyl-3-methoxypropionamide, which can solve human health hazards, equipment corrosion, and a large amount of strong alkali and other issues to achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
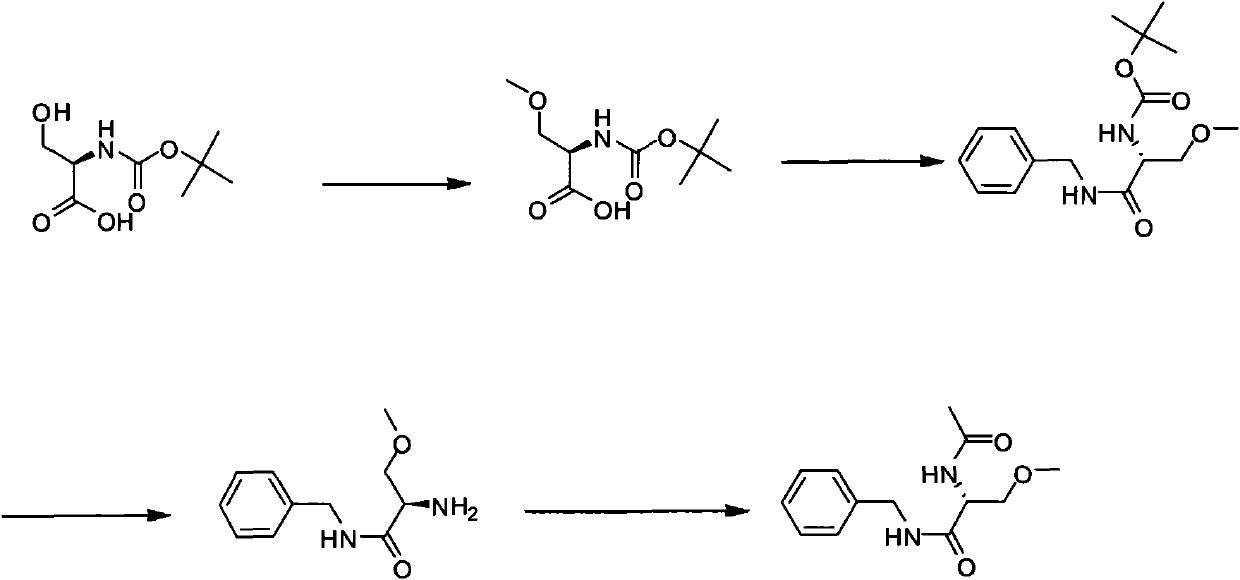
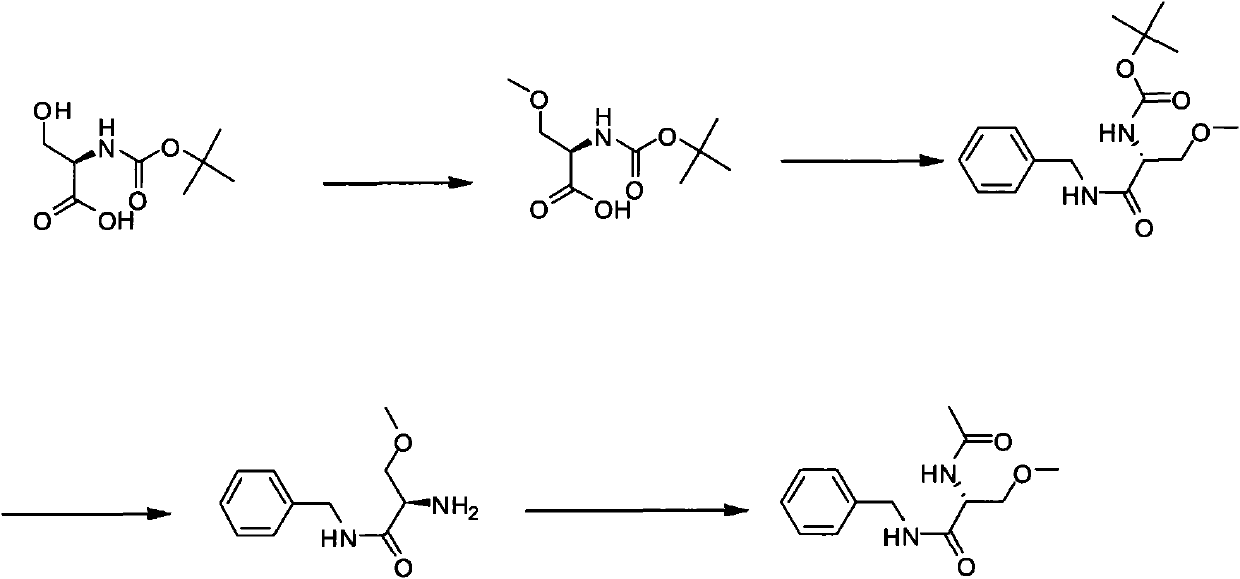
[0017] A new method for the synthesis of lacosamide comprising O-methylation of compounds of formula I in a single step reaction
[0018] Formula I
[0019] The compound of formula II is obtained
[0020] Formula II
[0021] O-methylation in the present invention is achieved by adding dimethyl carbonate to N-protected serine and reacting at 0-10°C for 10-24 hours in the presence of an organometallic compound.
[0022] The organometallic compound of the present invention is preferably an organolithium compound; the organolithium compound is preferably an alkyllithium compound, such as butyllithium, methyllithium or aryllithium; the organolithium compound is more preferably tert-butyllithium or n-butyllithium, particularly preferably is n-butyllithium.
[0023] figure 1 As shown, the synthetic method of lacosamide according to this embodiment specifically includes the following steps:
[0024] (1) Preparation of (R)-2-N-Boc-amino-3-methoxypropionic acid using dimethyl c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com