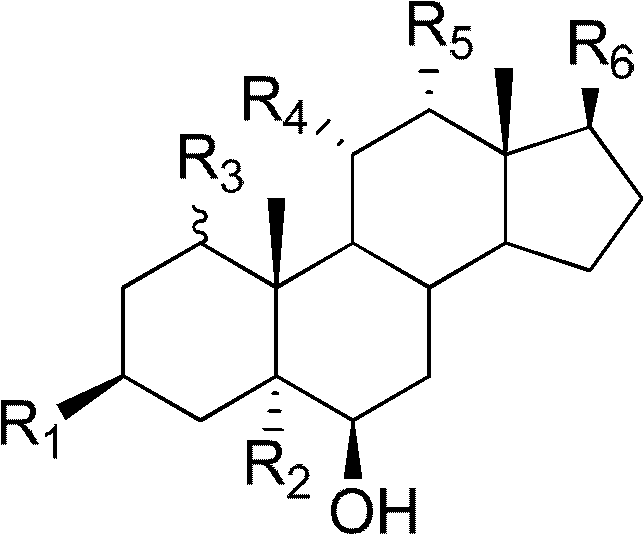
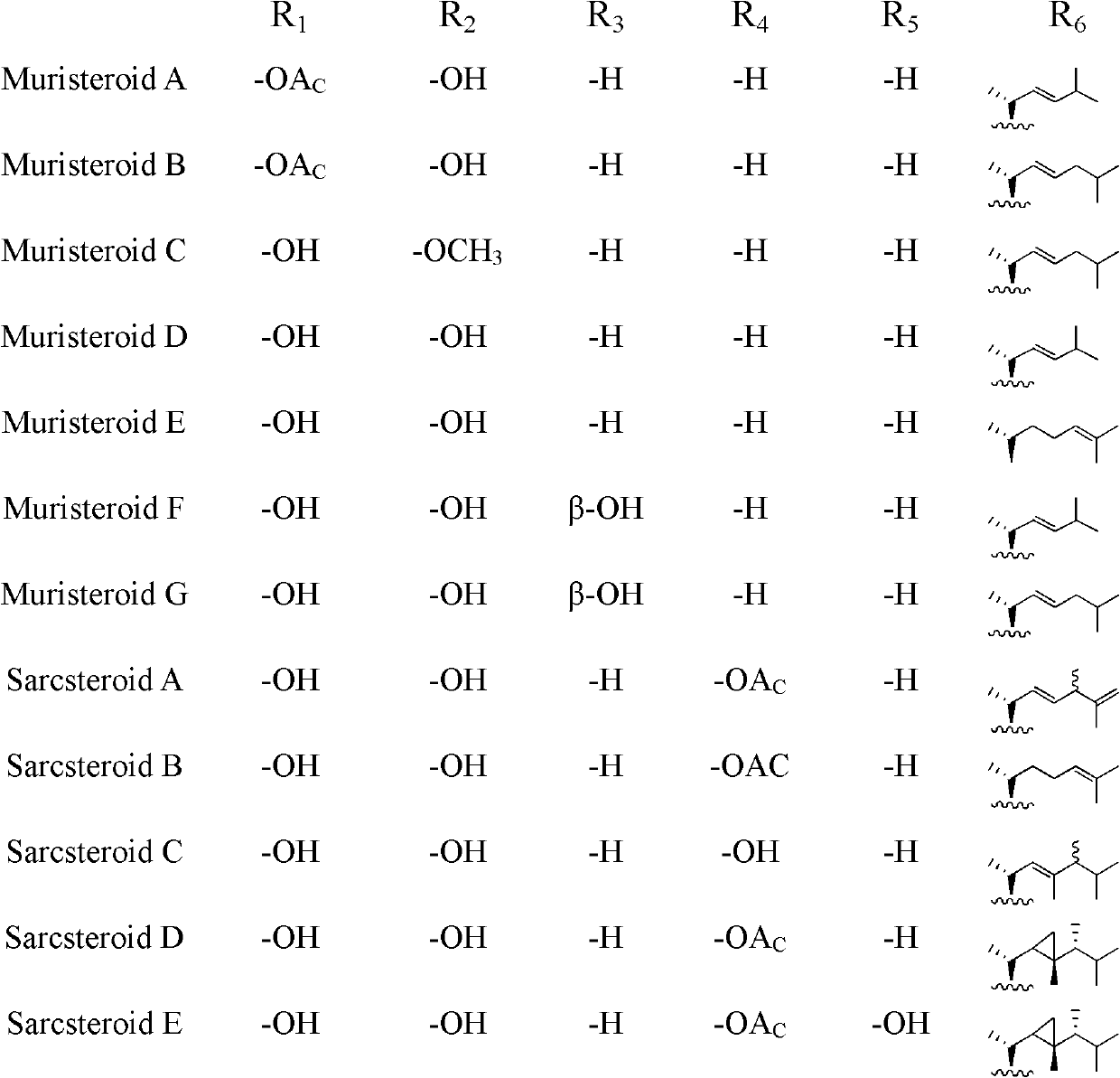
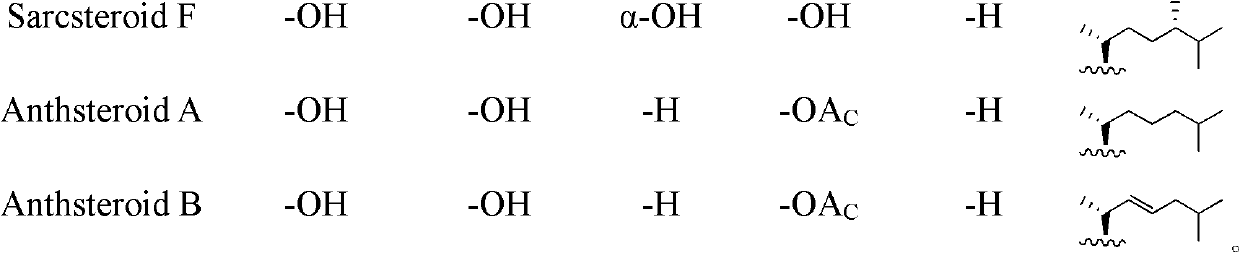
Polyhydroxy steroid compounds and purpose thereof
A technology for polyhydroxy steroids and compounds, which is applied to a class of polyhydroxy steroids and their application fields, and can solve problems such as undiscovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1. Preparation of Muristeroids A~G and 6 known compounds.
[0110] Take 1500g of Muriceopsis flavida from the waters near Sanya, Hainan, China, wash it, cut it into pieces, extract it ultrasonically with 5 times its weight of acetone, recover the acetone under reduced pressure until it has no acetone smell, disperse it with an equal volume of water, and extract it 6 times with ether , 1000ml each time, combined the extracts, recovered the ether and concentrated to dryness to obtain 12.5g of the total extract. The total extract is separated by normal phase silica gel chromatography (200-300 mesh), and the volume ratio is 80:1, 40:1, 20:1, 10:1, 5:1, 3:1, 2:1, 1 : 1, 1: 5, 1: 15 petroleum ether / ethyl acetate gradient elution, according to thin-layer plate monitoring, each fraction was collected according to polarity, and a total of 16 fractions Fr.1-Fr.16 were collected.
[0111] Part Fr.6 was separated by Sephandex LH-20 gel column chromatography (liquid petrole...
Embodiment 2
[0114] Embodiment 2. prepare Sarcsteroids A~F
[0115] Take 1642g of soft coral (Sarcophytum sp) from the waters near Beihai, Guangxi, China, wash it, cut it into pieces, extract it ultrasonically with 5 times its weight of acetone, recover the acetone under reduced pressure until there is no acetone smell, disperse it with an equal volume of water, and extract it with ether for 6 times, 1000ml each time, combined extracts, recovered ether and concentrated to dryness to obtain 21.6g of total extract. The total extract is separated by normal phase silica gel chromatography (200-300 mesh), and the volume ratio is 99:1, 79:1, 69:1, 59:1, 49:1, 39:1, 29:1, 25 :1, 22:1, 17:1, 15:1, 12:1, 9:1, 8:1, 7:1, 6:1, 5:1, 4:1, 3:1, 2:1 , 1:1, 1:2, 1:5, 1:10, 100% ethyl acetate petroleum ether / ethyl acetate for gradient elution, according to TLC monitoring, each fraction is from small to large according to polarity A total of 23 parts Fr.1~Fr.23 were collected and merged.
[0116] Part of ...
Embodiment 3
[0119] Embodiment 3. preparation Anthsteroids A~B
[0120] Take 2167g of Anthogorgia sp from the waters near Beihai, Guangxi, China, wash it, cut it into pieces, extract it ultrasonically with 5 times its weight of acetone, recover the acetone under reduced pressure until there is no acetone smell, disperse it with an equal volume of water, and extract it 6 times with ether , 2000ml each time, the combined extracts, recovered ether and concentrated to dryness. Suspend the total extract with 2000ml of water, extract 6 times with ether and n-butanol successively, combine and recover ether under reduced pressure to obtain 28 g of ether layer extract, combine n-butanol and recover n-butanol under reduced pressure to obtain n-butanol Alcoholic layer extract 5g. Ether layer extract (28g) was separated by normal phase silica gel column chromatography (200-300 mesh), and the volume ratio was 99:1, 49:1, 34:1, 29:1, 24:1, 19:1, 14 :1, 11:1, 9:1, 8:1, 7:1, 6:1, 5:1, 4:1, 3:1, 2:1, 1:1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com