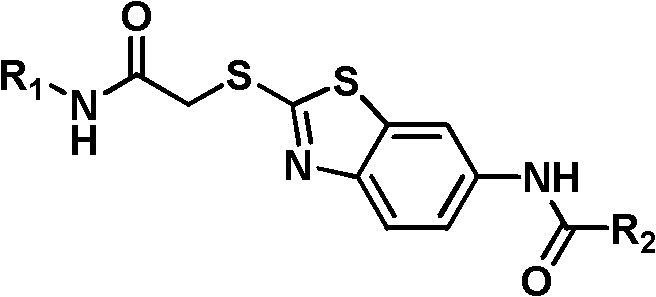
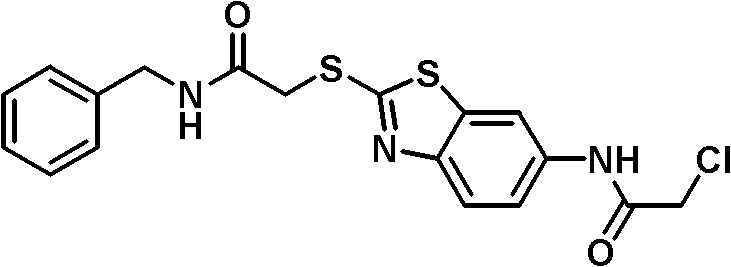
Emulsion of n-(2-(2-amino)oxyethylthiobenzothiazole-6-)-2-carboxamide derivative and its preparation method
A technology of benzothiazole and oxyethylthio, applied in the field of medicine, can solve the problems of killing tumor cells and damaging normal cells, low selectivity, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 CBTA emulsion
[0051] Put 12g of soybean lecithin for injection and 22g of glycerin for injection into a high-speed tissue grinder, add an appropriate amount of water for injection preheated to 80°C, and stir to make the phospholipids evenly dispersed in the water phase; another 500mg of CBTA is placed in a 200g preheated In the oil for injection, the oil phase is obtained after complete dissolution, and the temperature is lowered to 80°C for later use. Slowly add the oil phase to the water phase, and stir at high speed for 10 minutes (10000 rpm), so that the oil phase is evenly dispersed in the water phase, and white colostrum is prepared. Add water for injection to 1000ml of the colostrum, and place it in the high-pressure milk Repeated emulsification in the machine for 3 times until the average particle size is below 0.5 μm, filled with nitrogen, and sterilized by autoclaving to obtain CBTA intravenous emulsion. The measured encapsul...
Embodiment 2
[0052] The preparation of embodiment 2 CBTA emulsion
[0053] Dissolve and disperse 12g of soybean lecithin for injection, 3g of prolactin (F68) and 22g of glycerin for injection in an appropriate amount of water for injection preheated to 80°C to prepare a uniformly dispersed aqueous phase; add 100mg of CBTA to the preheated injection In 100g of oil, the oil phase is obtained after the complete dissolution, and the temperature is lowered to 80°C for later use. Slowly add the oil phase into the water phase, and stir at a high speed for 10 minutes (10000 rpm), to prepare light yellow colostrum. Add colostrum and water for injection to 1000ml, put it in a high-pressure milk homogenizer and homogenize it for 3 times until the average particle size reaches below 0.5μm, fill it with nitrogen, and sterilize it to obtain an intravenous emulsion. The measured encapsulation efficiency and particle size are: 87.47% and 324.8nm, respectively.
Embodiment 3
[0054] The preparation of embodiment 3 CBTA emulsion
[0055] Dissolve and disperse 15g of soybean lecithin for injection and 22g of glycerin for injection in an appropriate amount of water for injection preheated to 80°C to prepare a uniformly dispersed water phase; add 500mg of CBTA to 200g of preheated oil for injection, and dissolve completely to obtain Cool the oil phase to 80°C for later use. Slowly add the oil phase into the water phase, stir at high speed for 10 minutes (10000 rpm), and prepare white colostrum. Add colostrum and water for injection to 1000ml, put it in a high-pressure milk homogenizer and homogenize it for 3 times until the average particle size reaches below 0.5μm, fill it with nitrogen, and sterilize it to obtain an intravenous emulsion. The measured encapsulation efficiency and particle size are: 96.49% and 224.1nm, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com