Ionic liquid precursors and their supported mesoporous materials, synthesis and applications
A technology of ionic liquids and mesoporous materials, applied in the field of mesoporous materials, can solve problems such as weak force and desorption of ionic liquids, and achieve the effects of reducing usage, realizing recycling, and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Mechanism 1 is the reaction equation of the N-(3-triethoxysilylpropyl)-N-(3-propanesulfonic acid)-amine ionic liquid precursor prepared in Example 1, and the molecular formula is Si(OEt) 3 C 3 h 6 NH 2 C 3 h 6 SO 3 , referred to as SNSA. Mechanism 2 is the reaction equation of the 1-(3-triethylsilylpropyl)-3-(3-propanesulfonic acid)-4,5-dihydroimidazole ionic liquid precursor prepared in Example 2, and the molecular formula is Si (OEt) 3 C 3 h 6 NH 2 C 3 h 6 C 3 h 6 SO 3 , referred to as SSHI. Mechanism 3 is the reaction equation of the aldol condensation catalytic reaction in Examples 10-17. [Example 1] Synthesis of ionic liquid precursor N-(3-triethoxysilylpropyl)-N-(3-propanesulfonic acid)-amine, referred to as SNSA:
[0053] (1) Dissolve 18.00g (0.15mol) of 1,3-propane sultone in 30ml of tetrahydrofuran to form a solution, and slowly add the solution dropwise to 34.00ml (0.15mol) of 3-aminopropyltriethyl In oxysilane, reflux in oil bath at 60°C for...
Embodiment 2
[0055] [Example 2] Synthesis of ionic liquid precursor 1-(3-triethylsilylpropyl)-3-(3-propanesulfonic acid)-4,5-dihydroimidazole, referred to as SSHI:
[0056] (1) 5ml (0.018mol) of N-[3-(triethoxysilyl) propyl]-4,5-dihydroimidazole and 2.8g (0.023mol) of 1,3-propane sultone Put it into a 50ml flask, stir in an ice bath, 1,3-propane sultone dissolves, and the solution becomes viscous. Heat in an oil bath until the yellow viscous substance turns into a milky white solid, wash with ethyl acetate repeatedly, and finally dry to obtain the precursor product as a white powder. The structural formula is Si(OEt) 3 C 3 h 6 N 2 C 3 h 5 C 3 h 6 SO 3 , referred to as SSHI.
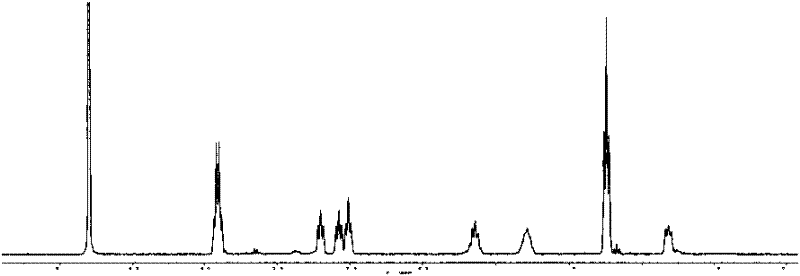
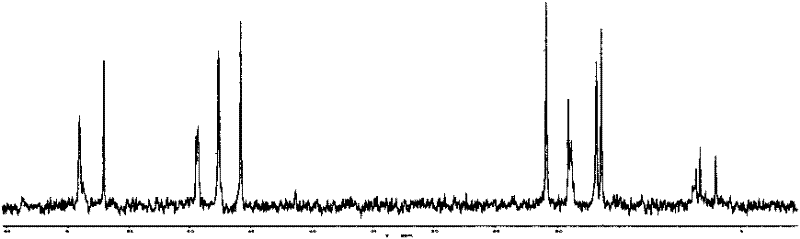
[0057] For the reaction equation, see Mechanism 2, and for NMR characterization, see Figure 4 and Figure 5 , 1 H NMR (D 2 O), δ(ppm)=0.75(t, 2H), 1.26(m, 9H, 3×CH 3 ), 1.77(m, 2H), 2.12(m, 2H), 2.96(t, 2H), 3.50(t, 2H), 3.65(t, 2H), 3.93(m, 6H, 3×CH 2 ), 3.99(s, 2×1H), 8.16(s, 1H); 13 C NMR (D 2 O), ...
Embodiment 3
[0058] [Example 3] MCM-41 loaded with tetraethylammonium hydroxide alkalized SNSSA ionic liquid was synthesized by sol-gel method, with a loading capacity of about 5%, named MCM-41-SNSA-5%-B1:
[0059] (1) Dissolve 0.22g of cetyltrimethylammonium bromide (CTAB) in 5ml of water, stir well and add 2.4ml of ammonia water, then add 0.95ml (4.3mmol) of tetraethyl orthosilicate (TEOS) and 0.077 g (0.23 mmol) of SNSA ionic liquid precursor was stirred evenly and placed in a stainless steel reaction kettle with a polytetrafluoroethylene liner, and crystallized at 100°C for 48 hours. After cooling, it was filtered, washed repeatedly with deionized water, and dried at room temperature.
[0060] (2) 0.3g of the product prepared in step (1) is placed in 5ml of tetraethylammonium hydroxide solution with a concentration of 0.1mol / L, filtered evenly after stirring, repeatedly washed with deionized water to neutrality, and dried at room temperature. Instant product.
[0061] The XRD of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com