Process for purifying typhoid Vi polysaccharide
A polysaccharide and typhoid technology, applied in the direction of antibacterial drugs, etc., can solve the problems of injury to production personnel, clean removal of phenol, and damage to the ecological environment, and achieve the effects of protecting the environment, improving production efficiency, and avoiding large-scale use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
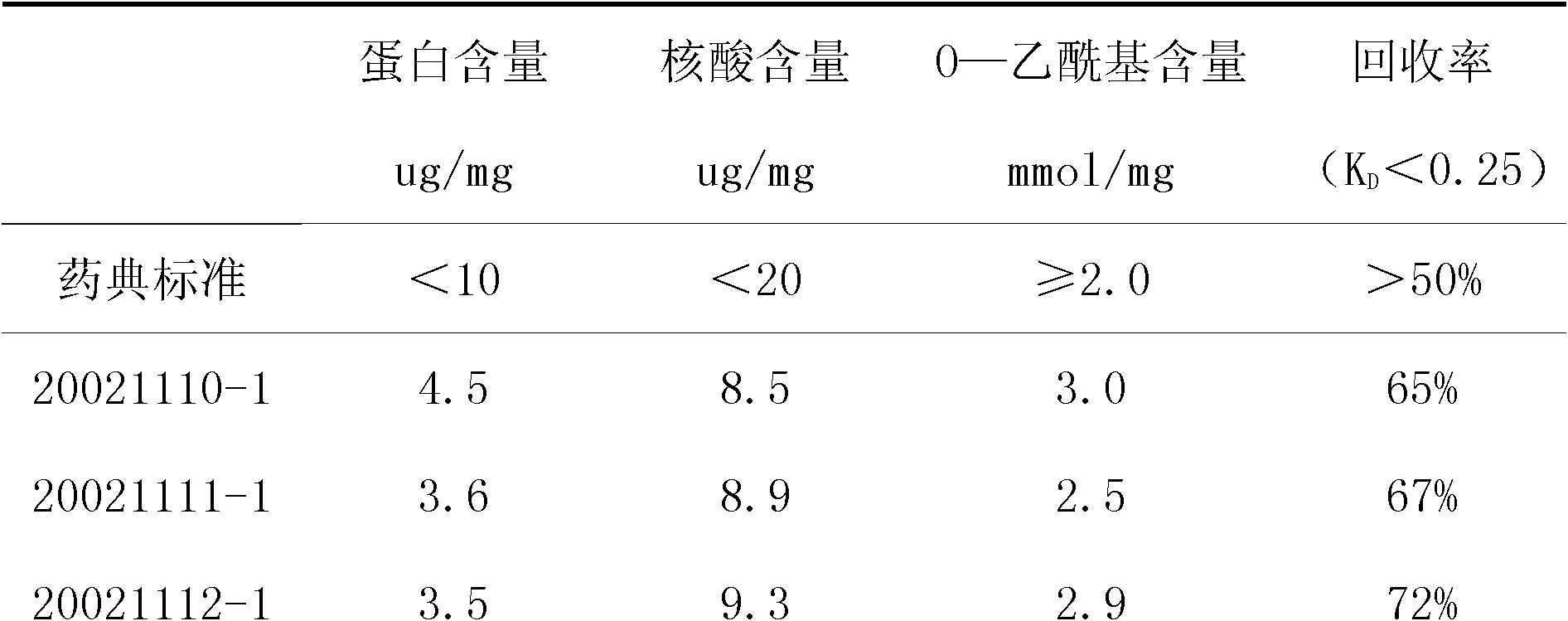
[0077] The typhoid Vi polysaccharide purification process provided by the invention comprises the following steps:
[0078] Step 1: The sterilized culture solution is centrifuged to remove bacteria and collect the supernatant.
[0079] Step 2: Add cetyltrimethylammonium bromide to the supernatant to a final concentration of 0.06% to 0.10%, mix well, and store at 2 to 8°C for 4 to 24 hours to form a precipitate;
[0080] Step 3: collect the precipitate by centrifugation, dissolve the precipitate with 0.5mol / L sodium chloride solution, and stir for 2 hours to dissociate the polysaccharide from cetyltrimethylammonium bromide;
[0081] Step 4: Add 95% ethanol to the dissociation solution until the final ethanol concentration is 30%, store at 2-8°C for 3-24 hours, and collect the supernatant by centrifugation;
[0082] Step 5: Filter the supernatant through an asbestos-free clarification filter plate (K700), and the pressure during filtration is 0.03-0.05Mpa;
[0083] Step 6: Fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com