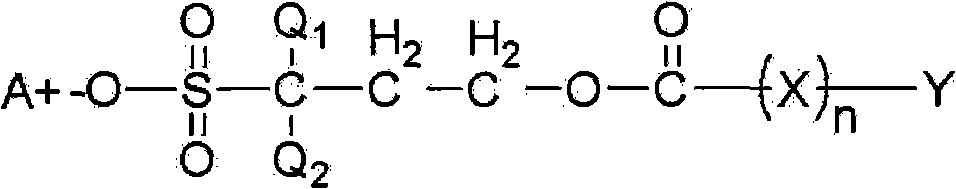Photoacid generator, its preparation method and resist composition containing the photoacid generator
A technology of photoacid generators and compounds, applied in the preparation of sulfonates, photosensitive materials for optomechanical equipment, organic chemistry, etc., can solve problems such as short diffusion distance, improve LWR) characteristics, and low diffusion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0208] A resist solution was prepared in the same manner as in Comparative Example 1, except that 3 parts by weight of benzoic acid-2,2-difluoro-2 represented by Formula B in Reaction Formula 4 prepared in Synthesis Example 1 was used. -Sulfo-propyl ester diphenylmethylphenylsulfonium salt as photoacid generator. The properties of the resist solution were evaluated.
Embodiment 2
[0210] A resist solution was prepared in the same manner as in Comparative Example 1, except that 5 parts by weight of benzoic acid-2,2-difluoro-2 represented by Formula B in Reaction Formula 4 prepared in Synthesis Example 1 was used. -Sulphopropyl ester diphenylmethylphenylsulfonium salt as photoacid generator. The properties of the resist solution were evaluated.
Embodiment 3
[0212] A resist solution was prepared in the same manner as in Comparative Example 1, except that 7 parts by weight of benzoic acid-2,2-difluoro-2 represented by Formula B in Reaction Formula 4 prepared in Synthesis Example 1 was used. -Sulphopropyl ester diphenylmethylphenylsulfonium salt as photoacid generator. The properties of the resist solution were evaluated.
[0213] Properties of Comparative Example 1 and Examples 1 to 3, such as sensitivity, resolution and LWR, were evaluated, and the results are listed in Table 1 below.
[0214] In the case of LWR, the pattern roughness of a 0.10-μm line-and-space (L / S) pattern formed after development was observed, and the degree of improvement in LWR was graded from 1 to 5, Comparative Example 1 The pattern obtained in the grade 1. A larger value indicates better LWR characteristics.
[0215] In the case of sensitivity, the exposure amount for forming a 0.10-μm line-and-space (L / S) pattern with a line width of 1:1 is specified ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com