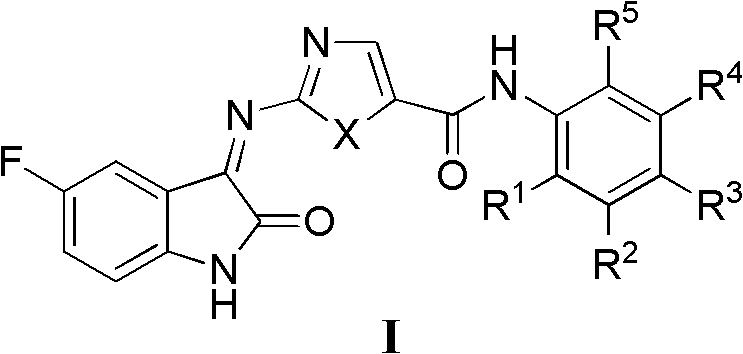
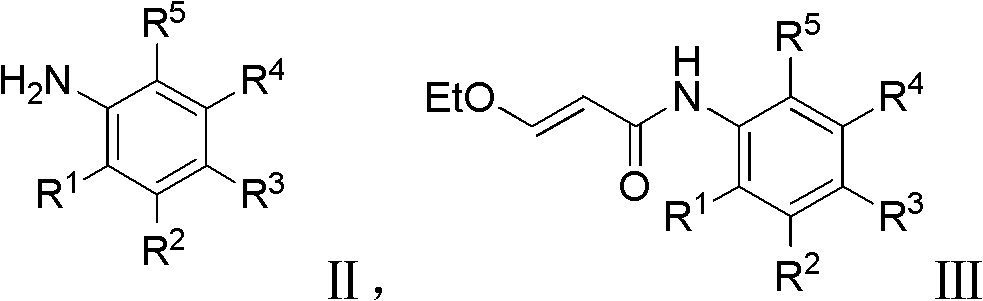
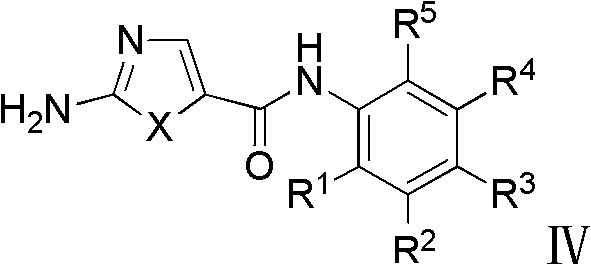
3-heterocycle schiff base-5-fluorine-indole-2-ketone compounds, preparation method thereof and application thereof
A ketone compound, Schiff base technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128]
[0129] 1A(E)-N-Phenyl-3-ethoxyacrylamide
[0130] Under cooling in an ice bath, dissolve aniline (9.3g, 0.1mol) and pyridine (16.2mL, 0.15mol) in anhydrous THF (150mL), and slowly add 3-ethoxyacryloyl chloride dropwise at a temperature of 0-5°C (15.0g, 0.11mol), after the dropwise addition, the temperature was naturally raised to room temperature, and the reaction was continued for 2h. Add 1N dilute hydrochloric acid (25mL) under ice-cooling, then add water (50mL) to dilute, concentrate under reduced pressure to a viscous oil, add toluene (70mL), stir at 50°C for 5min, cool to 0°C, and continue stirring for 1h. Filter, wash with water, and dry to obtain 15.3 g of solid, with a yield of 80.1%. MSI-MS: 192.1[M+H] + ; 1 H NMR (300Hz, CDCl 3 ) 1.29 (t, 3H, J=7.5Hz, CH 3 CH 2 ), 3.94(q, 2H, J=7.5Hz, CH 3 CH 2 ), 5.53(d, 1H, J=12.3Hz, =CH-CO), 7.30-7.33(m, 1H, Ar-H), 7.50-7.53(m, 2H, Ar-H), 7.55(d, 1H , J=12.3Hz,=CH-O), 7.63-7.66 (m, 2H, Ar-H), 9.67 (s, 1H, CONH...
Embodiment 2
[0136]
[0137] 2A(E)-N-(2-methyl-6-chlorophenyl)-3-ethoxyacrylamide
[0138] Referring to the synthetic method of 1A, the yield is 68.5%. MSI-MS: 240.1[M+H] + ; 1 H NMR (300Hz, CDCl 3 )
[0139] 1.28(t, 3H, J=7.5Hz, CH 3 CH 2 ), 2.16(s, 3H, Ph-CH 3 ), 3.94(q, 2H, J=7.5Hz, CH 3 CH 2 ), 5.59 (d, 1H, J=12.6Hz, =CH-CO), 7.08-7.24 (m, 2H, Ar-H), 7.32 (m, 1H, Ar-H), 7.49 (d, 1H, J =12.6Hz, =CH-O).
[0140] 2B 2-Amino-N-(2-methyl-6-chlorophenyl)thiazole-5-amide
[0141] Referring to 1B synthesis method, the yield is 86.7%. MSI-MS: 268.1[M+H] + ; 1 H NMR (300Hz, DMSO-d6)
[0142] 2.19(s, 3H, CH 3 ), 7.08-7.26(m, 2H, Ar-H), 7.30-7.43(m, 1H, Ar-H), 7.61(s, 2H, NH 2 ), 7.86 (s, 1H, thiozole-H), 9.69 (s, 1H, CONH).
[0143] I-2 2-(5-fluoroindol-2-one-3-methylene)amino-N-(2-methyl-6-chlorophenyl)thiazole-5-amide
[0144] Referring to the synthesis method of I-1, the yield is 21.0%. MSI-MS: 415.1[M+H] + ; 1 H NMR (300Hz, DMSO-d6)
[0145] 2.20(s, 3H, CH 3 ), 7.24-...
Embodiment 3
[0147]
[0148] 3A(E)-N-(4-fluorophenyl)-3-ethoxyacrylamide
[0149] Referring to the synthetic method of 1A, the yield is 70.3%. MSI-MS: 210.2[M+H] + ; 1 H NMR (300Hz, CDCl 3 )
[0150] 1.27(t, 3H, J=7.1Hz, CH 3 CH 2 ), 3.95 (q, 2H, J=7.1Hz, CH 3 CH 2), 5.50 (d, 1H, J=12.0Hz, =CH-CO), 7.07-7.16 (m, 2H, Ar-H), 7.48 (d, 1H, J=12.0Hz, =CH-O), 7.59 -7.66 (m, 2H, Ar-H), 9.74 (s, 1H, CONH).
[0151] 3B 2-Amino-N-(4-fluorophenyl)thiazole-5-amide
[0152] Referring to 1B synthesis method, the yield is 88.6%. MSI-MS: 238.1[M+H] + ; 1 H NMR (300Hz, DMSO-d6)
[0153] 7.10-7.19(m, 2H, Ar-H), 7.60(s, 2H, NH 2 ), 7.63-7.67 (m, 2H, Ar-H), 7.86 (s, 1H, thiozole-H), 10.11 (s, 1H, CONH).
[0154] I-3 2-(5-fluoroindol-2-one-3-methylidene)amino-N-(4-fluorophenyl)thiazole-5-amide
[0155] Referring to the synthesis method of I-1, the yield is 35.6%. MSI-MS: 385.1[M+H] + ; 1 H NMR (300Hz, DMSO-d6)
[0156] 7.15-7.19(m, 2H, Ar-H), 7.33-7.36(m, 1H, Ar-H), 7.63-7.71(m, 2H, Ar-H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com