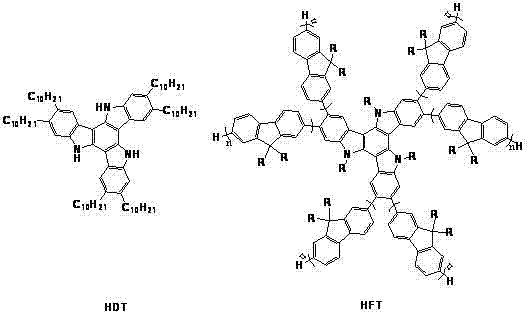
All-alkyl substituted triindene cyclotrimeric indole derivatives and preparation method thereof
A technology for alkylation of trimerindole and trimerindole, which is applied in chemical instruments and methods, organic chemistry, luminescent materials, etc., can solve problems such as limited research and difficult synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
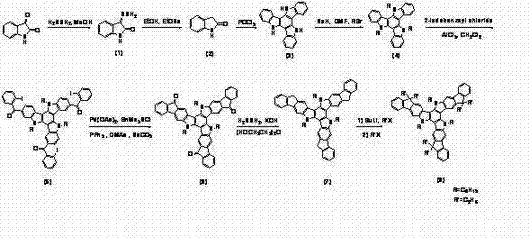
[0038] Isatin-3-hydrazone (1) preparation of
[0039] Isatin (25.00 g, 170.00 mmol) was dissolved in 200 ml of methanol, 85% hydrazine hydrate (19.40 g, 85%, 515 mmol) was added, heated to reflux for 60 minutes and then cooled, the precipitated solid was suction filtered and dried to obtain yellow Solid (25.15 g, 91.2 %). M.p.: 225°C (decomposition); literature value: 215-219°C.
[0040] 1H-indol-2-one (2) preparation of
[0041] Add sodium ethoxide solution (10.00 g sodium, 250 ml absolute ethanol) dropwise into solid isatin-3-hydrazone (1) (25.00 g, 155.13 mmol), after heating to reflux for 4 hours, the reaction solution was poured into water, extracted with dichloromethane to obtain an organic phase, washed with water, dried with anhydrous magnesium sulfate, spin-dried, and washed with absolute ethanol / petroleum Ether was recrystallized and sucked dry to obtain a white solid (12.40 g, 60.7 %). M.p.: 121-123°C; literature value: 125-127°C.
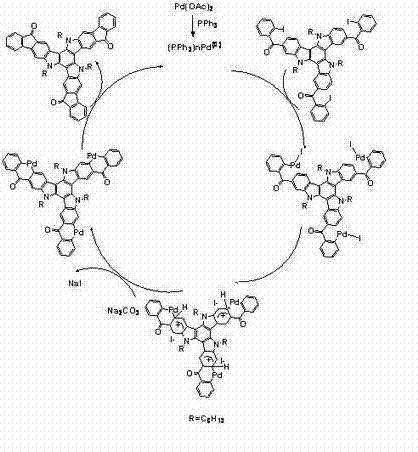
[0042] trimindole (3) prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com