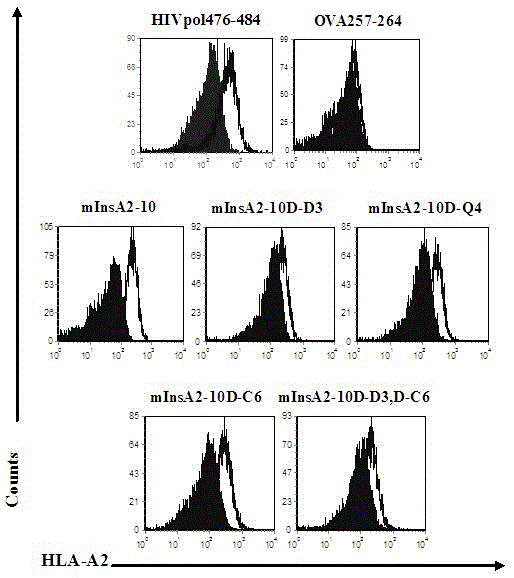
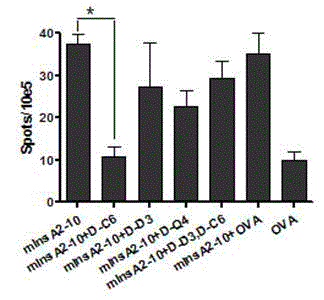
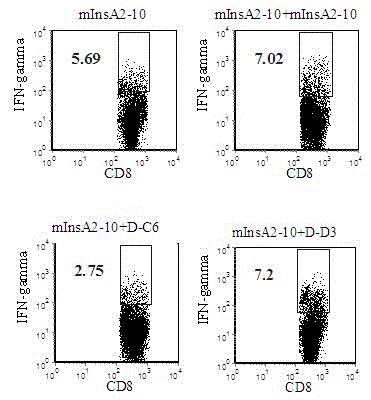
Insulin chain A human leukocyte antigen (HLA)-A*0201 restrictive cytotoxic T lymphocyte (CTL) epitope altered peptide ligand and application thereof
A technology of HLA-A and insulin, applied in the field of biomedicine, achieves the effect of inhibiting CTL response, safe use, and good development and application prospects
Inactive Publication Date: 2013-02-06
ARMY MEDICAL UNIV
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
CD4 freshly isolated from islets of NOD mice with T1D alone + Adoptive transfer of T cells to NOD scid mice did not induce T1D; however, the islets infiltrated CD8 + T cells, but can knock out CD4 + T1D is rapidly induced in NOD or NOD scid mice of T cells, whereas knockout of CD8 + T cells or CD8α-knockout NOD mice also exhibit T1D resistance
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0016] In order to make the object, technical solution and advantages of the present invention clearer, preferred embodiments of the present invention will be described in detail below with reference to the accompanying drawings.
[0017] 1. Design and preliminary screening of APL
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses an insulin chain A human leukocyte antigen (HLA)-A*0201 restrictive cytotoxic T lymphocyte (CTL) epitope altered peptide ligand (APL) which is obtained by replacing sixth amino acid L-Cys in insulin chain A HLA-A*0201 restrictive natural CTL epitope mInsA2-10 of humanized non-obese diabetic (NOD) mice with D-Cys. The amino acid sequence of the APL is shown as SECIDNo.1L; the natural CTL epitope has high cross reactivity with human epitope, and the APL has an obvious characteristic of inhibiting natural epitope specificity CTL response in the extracorporal cellar immunefunction experiment of the humanized NOD mice and the peptide has the characteristics that the peptide is easy to prepare and purify in a large scale and can be used safely, so that the APL can be independently applied to the development of I-type diabetes mellitus therapeutic antagonist peptide vaccine or is combined with other immunodominance autoantigen epitopes or APL to be applied to the development of I-type diabetes mellitus therapeutic antagonist peptide vaccine, and has potential and good development and application prospect in the field of human I-type diabetes mellitus treatment.
Description
technical field [0001] The invention belongs to the field of biomedicine and relates to an antigen epitope modified peptide ligand (altered peptide ligand, APL), in particular to the HLA-A*0201 restricted CTL epitope APL of insulin A chain (mInsA). Background technique [0002] Type I diabetes (Type 1 diabetes, T1D) is a type of autoimmune disease mediated by T lymphocytes and characterized by the elimination of insulin-producing β-cells, which mostly occurs in children and adolescents. At the same time, since hyperglycemia may further cause eye, blood, cardiovascular and nervous system diseases, it is also very important to prevent and control pathological autoimmune symptoms such as pancreatic tissue destruction. At present, T1D is mainly treated with exogenous insulin replacement, which is expensive and has many side effects. Therefore, it is urgent to find an interventional treatment strategy that can effectively down-regulate the autoimmune state. [0003] In recent ye...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07K7/06A61K39/00A61P3/10
Inventor 王莉舒驰陈晓玲牛微吴玉章
Owner ARMY MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com