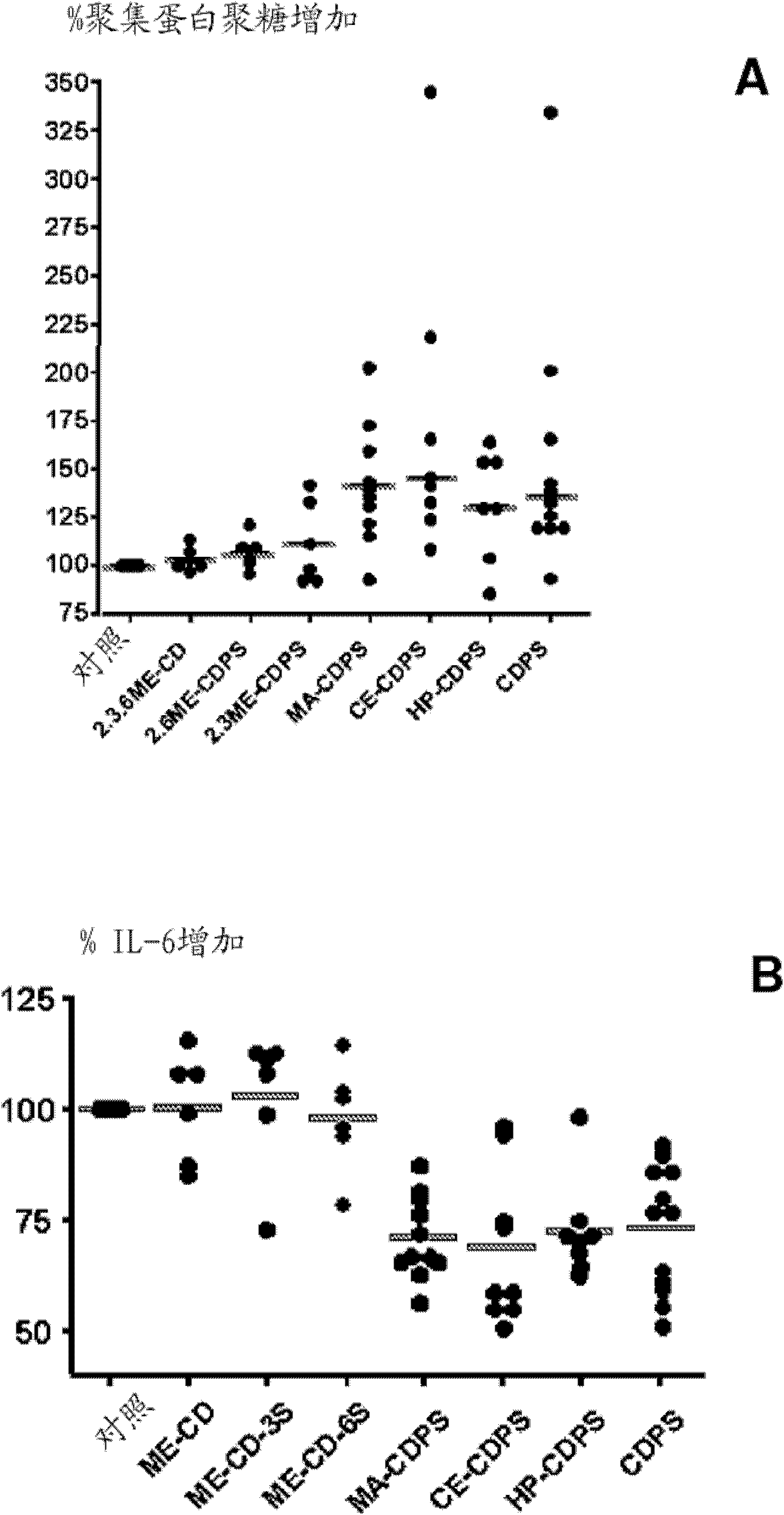
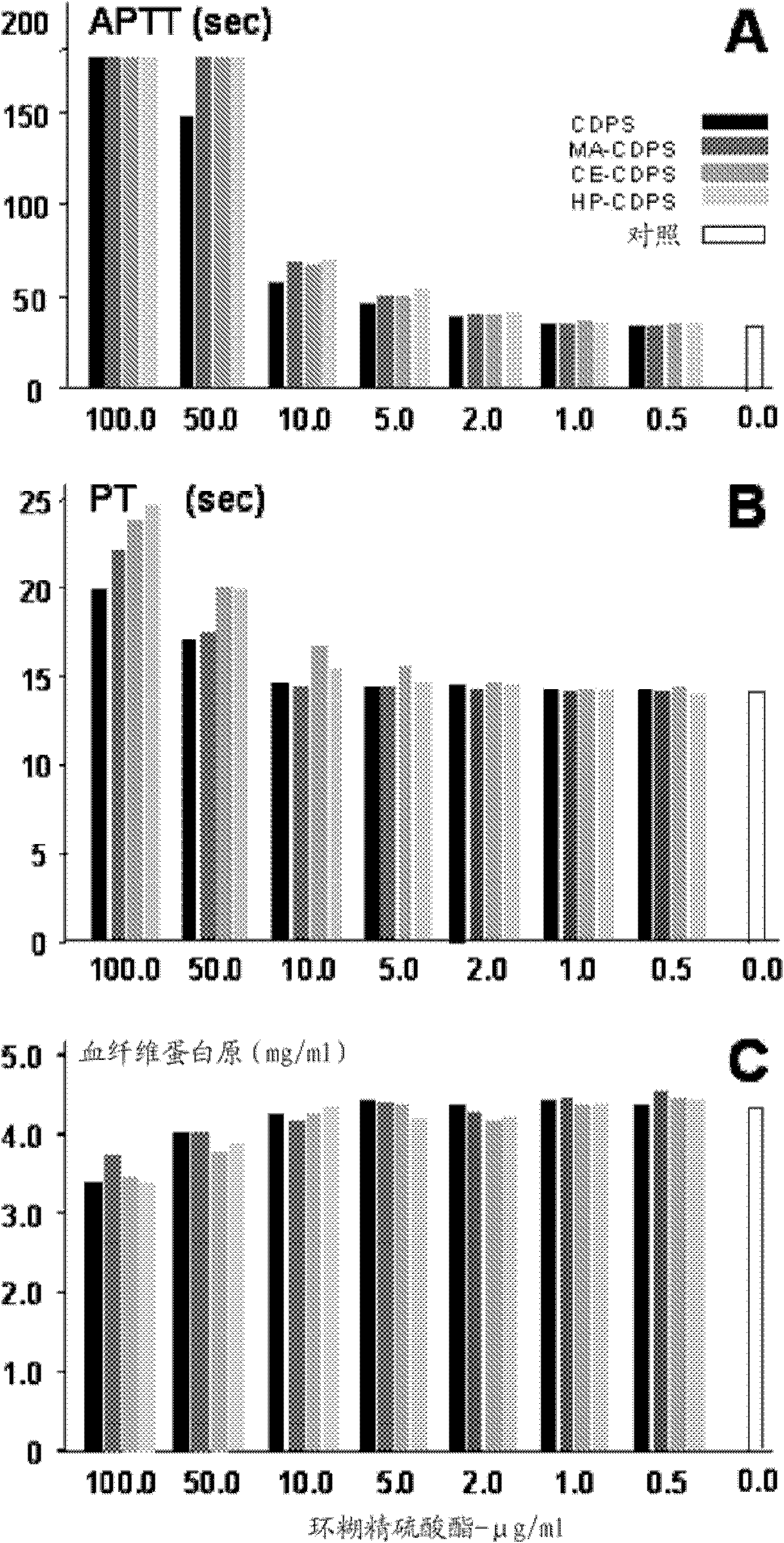
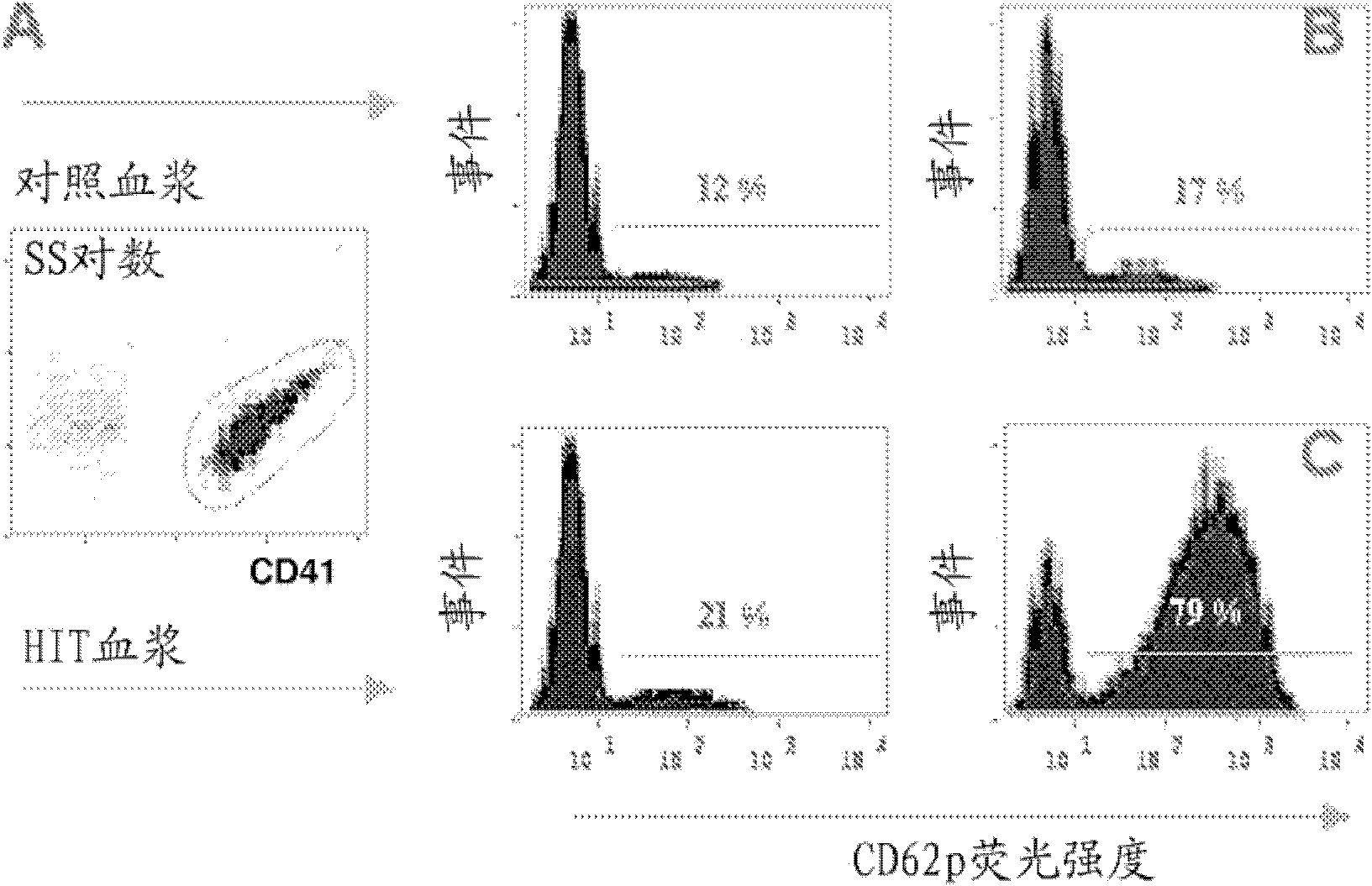
Carboxyalkylcyclodextrin polysulfates used as pharmaceuticals
A cyclodextrin and sulfate technology, which can be used in drug combinations, antipyretics, pharmaceutical formulations, etc., can solve problems such as reduced anticoagulant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 - Sulfation of Carboxyethyl-β-Cyclodextrin
[0065] Heat pyridine sulfonate (sulfur trioxide pyridine complex) to 70-80°C in a water bath. Pyridine sulfonate (1000 mL) was added to a sidearm vessel which was mechanically stirred and maintained at 80°C with a water bath. Carboxyethyl-β-cyclodextrin with a degree of substitution of 3 (100 g) from Cyclolab Ltd. (Hungary) was slowly added with rapid stirring. The mixture was maintained at 80°C with stirring for 2.5 hours, then water (500 mL) was added. The preparation of embodiment 2-carboxyethyl sulfate-beta-cyclodextrin sodium salt
[0066] The reaction mixture obtained in Example 1 was poured into methanol (7500 mL) containing sodium acetate (750 g) while stirring rapidly. The resulting precipitate was isolated by filtration using a Buchner funnel, washed with methanol (2000 mL), air-dried, and then dried under vacuum. Then, dissolve the precipitate (carboxyethylsulfate-β-cyclodextrin sodium salt) in water ...
Embodiment 3
[0067] Activated carbon treatment of embodiment 3-carboxyethyl cyclodextrin polysulfate sodium salt
[0068] 2-Carboxyethyl-β-cyclodextrin polysulfate sodium salt (hereinafter referred to as "CE-CDPS") obtained in Example 2 was dissolved in water at a rate of 300 mg / mL. Add activated carbon (10g) per 100g of cyclodextrin polysulfate sodium salt. The mixture was stirred at room temperature for 30 minutes and filtered several times, including finally using a 0.2 μm filter, to remove the charcoal. The filtered cyclodextrin compound solution was slowly added to 5 volumes of stirring methanol. The mixture was stirred for 10 minutes. The resulting product was isolated by filtration, washed with methanol, and dried under vacuum. The elemental analysis data are shown in Table 1.
Embodiment 4
[0069] Comparative Elemental Analysis of Example 4-β-Cyclodextrin Derivatives
[0070] 2,3,6-tri-O-methyl-β-cyclodextrin (ME-CD) and 2,6-di-O-methyl-3-sulfate-β-cyclodextrin (ME-CD- 3-S) was purchased from Cyclolab Ltd (Hungary). 2,3-Di-O-methyl-6-sulfate-β-cyclodextrin (ME-CD-6-S was supplied by Regis Technologies Inc. (Morton Grove, IL, USA). 2-Hydroxypropyl -β-cyclodextrin (HP-CDPS) was provided by Sigma Chemical Company, and 6-monodeoxy-6-monoamino-β-cyclodextrin (MA-CDPS) was from Cyclolab Ltd. The elemental analysis data are shown in Table 1, Compare with the data of the purified 2-carboxyethyl-β-cyclodextrin polysulfate sodium salt obtained in Example 3.
[0071]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com