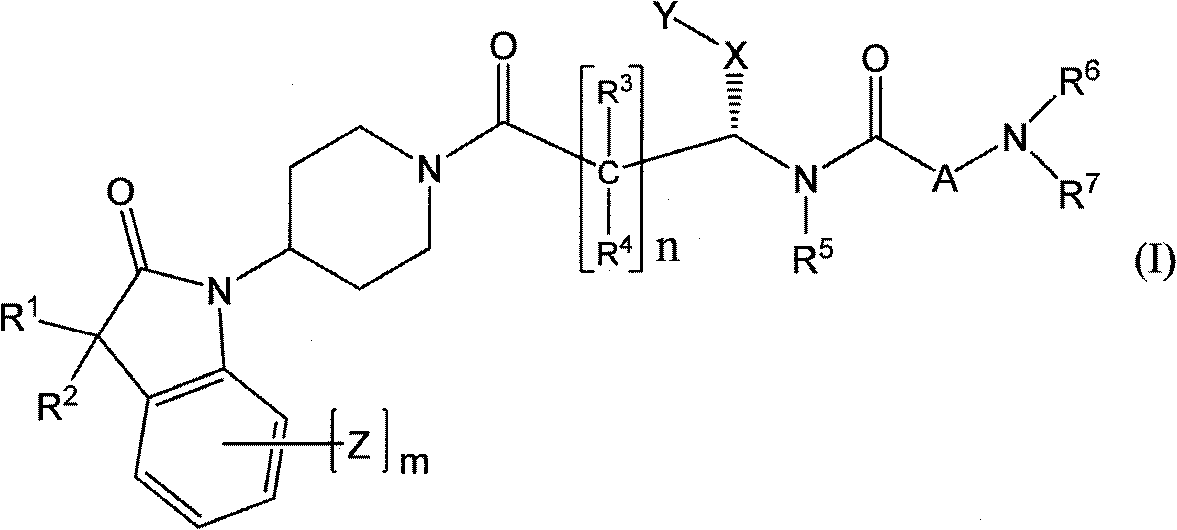
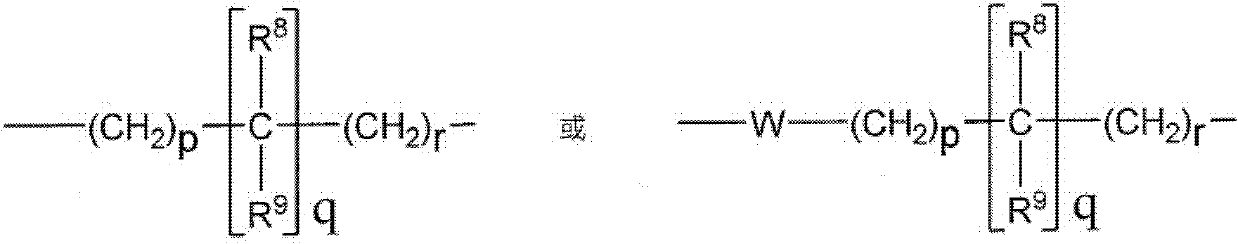
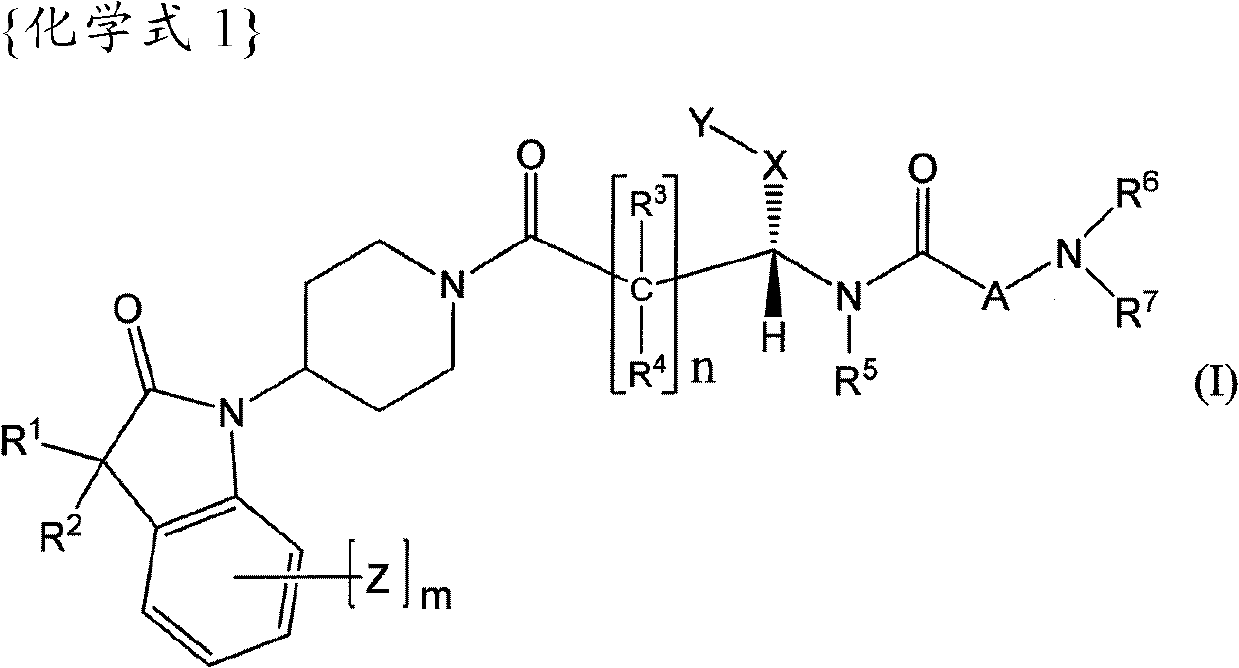
Oxyindole derivatives with motilin receptor agonistic activity
A hydroxyl and alkyl technology, applied in the field of novel oxindole derivatives or pharmaceutically acceptable salts thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0174] The preparation of the compounds of formula I according to the invention can be carried out in sequential or convergent synthetic routes.
[0175] Synthetic details for the preparation of the compounds of formula I of the present invention are shown in the following reaction schemes in a sequential manner.
[0176] The term "standard peptide coupling reaction conditions" is used repeatedly herein to refer to inert solvents such as DMF and methylene chloride in the presence of catalysts such as HOBT and / or such as diisopropylethylamine or triethylamine Acid activators such as EDC, DCC, HBTU, and BOP are used to couple carboxylic acids with amines in the presence of bases.
[0177] The term "PG1" used hereinafter refers to an amino-protecting group selected from typical amino-protecting groups described in the literature [Protective Groups in Organic Synthesis edited by T.W. Greene et al. (John Wiley & Sons, 1999). Typical amino-protecting groups include benzyl, CBZ, FMO...
Embodiment 1
[0263] (R)-N-((S)-1-(4-(3,3-Dimethyl-2-oxoindolane-1-yl)piperidin-1-yl) -1-oxo-4-phenylbutan-2-yl)piperidine-3-carboxamide
[0264]
[0265] step 1
[0266] 1-(1-Benzylpiperidin-4-yl)-3,3-dimethylindolin-2-one
[0267] To a stirred solution of N,N-diisopropylethylamine (28.9 mL, 205 mmol) and dry tetrahydrofuran (446 mL) cooled to -78°C was added dropwise n-butyllithium (82.3 mL, 205 mmol) under nitrogen atmosphere. ). After stirring the reaction mixture for 10 minutes at -78°C, 1-(1-benzylpiperidin-4-yl)indolin-2-one (21.0 g, 68.0 mmol, Tetrahedron Communications, 2004 , 50, 8535-8537) in THF (258 mL). The reaction mixture was stirred for an additional 30 minutes and iodomethane (12.8 mL, 205 mmol) was added. The reaction mixture was warmed to room temperature and stirred overnight. The reaction mixture was cooled to 0 °C, and aqueous ammonium chloride solution was added to quench the reaction. The aqueous layer was extracted with ethyl acetate (x3). The org...
Embodiment 2~16
[0300]
[0301] The following examples were prepared following a procedure similar to that described in step 5 of Example 1, using the appropriate precursor in place of (R)-1-(tert-butoxycarbonyl)piperidine-3-carboxylic acid , i.e. Examples 2-16.
[0302] {Table 4-A}
[0303]
[0304]
[0305] {Table 4-B}
[0306]
[0307]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com