Application of compound in preparing anti-mammary cancer medicament aiming at CCL (CC chemokine ligand) 18 target
An anti-breast cancer, compound technology, applied in the direction of anti-tumor drugs, drug combinations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
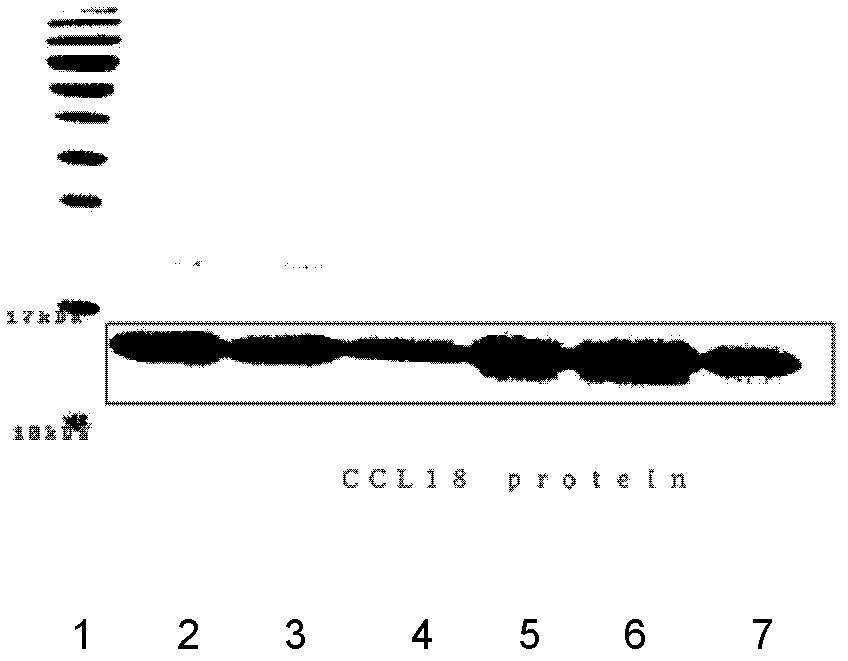
[0027] Embodiment 1: SDS-PAGE experiment of the purified CCL18
[0028] The CCL18 gene encodes a sequence of 89 amino acids, and after the 20 amino acid residues at the amino terminal are excised, the mature protein contains 69 amino acids. The human CCL18 protein can contain 69 amino acids, and there is also a 68 amino acid form (with alanine missing at the carboxy terminus). In previous work, we have cloned, expressed, and purified mature human CCL18, its SDS-PAGE is shown as figure 1 Shown, where band 1 is molecular weight marker; bands 2-7 are CCL18 protein. CCL18 is also commercially available as PeproTech 96-300-34-100ug Recombinant Human MIP-4 (CCL18).
Embodiment 2
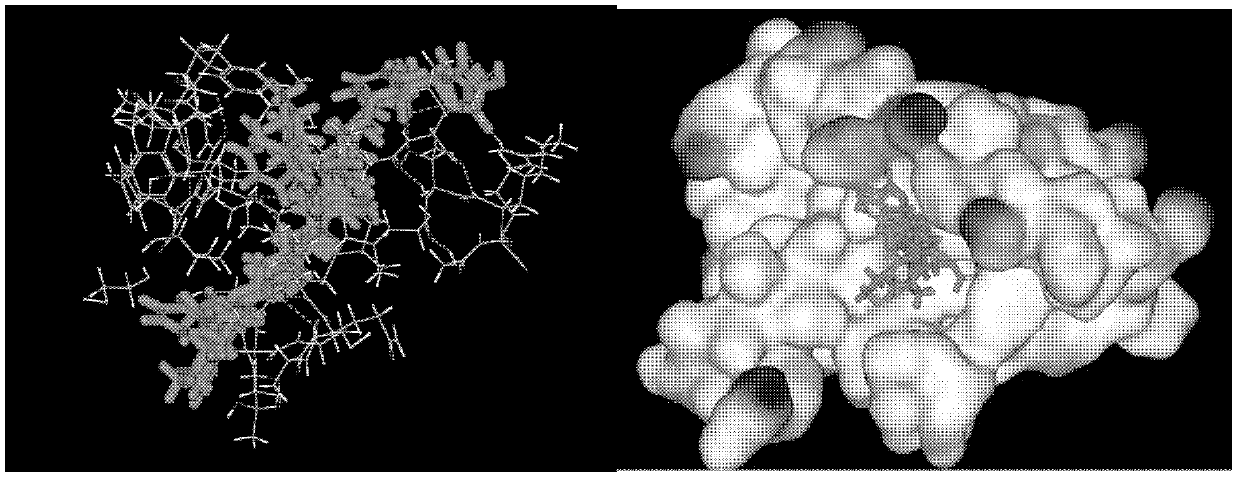
[0029] Example 2: Screening Inhibitors from Existing Small Molecule Libraries Using Computer Simulation Molecular Docking
[0030] Subsequently, the three-dimensional space structure of human CCL18 was established by homology modeling method, and the inhibitor was screened from the existing small molecule library by computer simulation molecular docking method. For molecules with higher scores, further use the computer to observe the interaction with amino acids in CCL18, especially the hydrogen bond, the interaction between positive and negative charges, л-лstacking, and the interaction between hydrophobic groups. Then multiple active small molecules are selected to overlap in the binding site to establish a pharmacophore, and then computer docking is carried out on the basis of this pharmacophore to obtain a series of analogs. In the preliminary screening, we obtained 15 compounds with high scores, active multiple small molecules overlapped in the binding site by computer do...
Embodiment 3
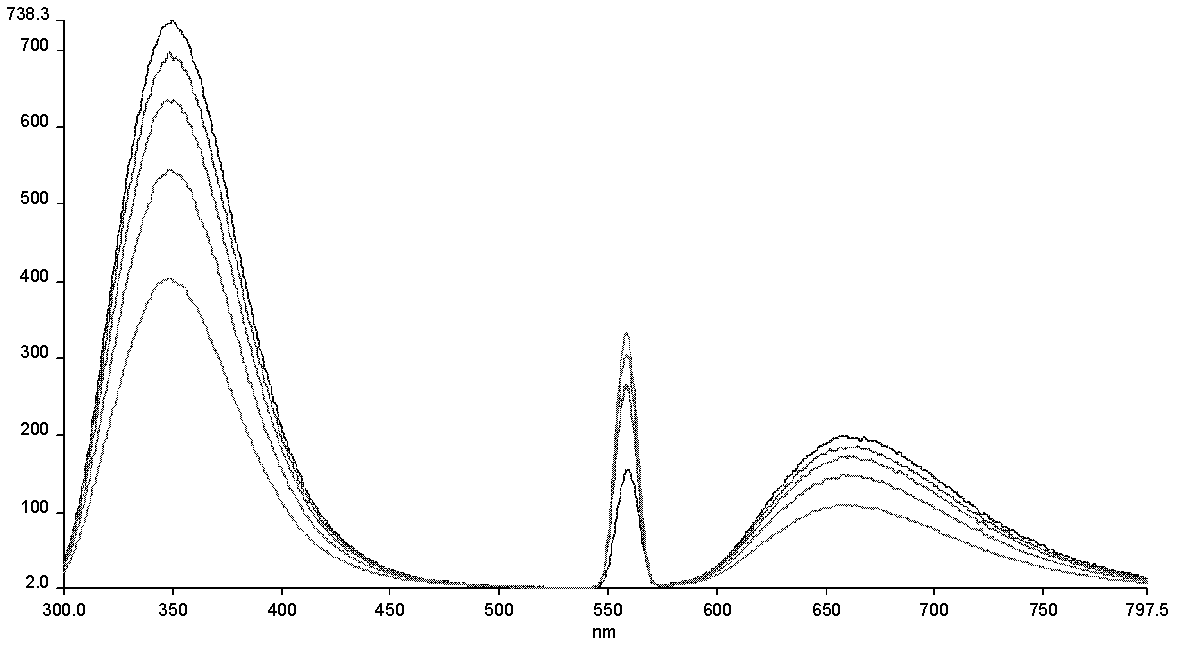
[0031] Example 3: Interaction experiment between glycine ester with protective group and protein
[0032] The interaction between glycine esters with protective groups and proteins was further studied by fluorescence chromatography, ITC (isothermal titration calorimetry), CD (circular dichroism), SPR (surface plasmon resonance) and other methods. The binding constant of glycine ester with protective group to CCL18 was measured by SPR method in the range of hundreds of nanomoles. There are multiple amino acids with fluorescent group side chains in the protein sequence of CCL18 (including W=Tryptophan tryptophan, Y=Tyrosine tyrosine), among which there are multiple binding sites between CCL18 and compounds, so through the fluorescence spectrum The conformational change of CCL18 before and after binding the compound can be compared. Fluorescence chromatographic titration changes of glycine ester with protective group and CCL18 protein as follows image 3 shown. The above exper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com