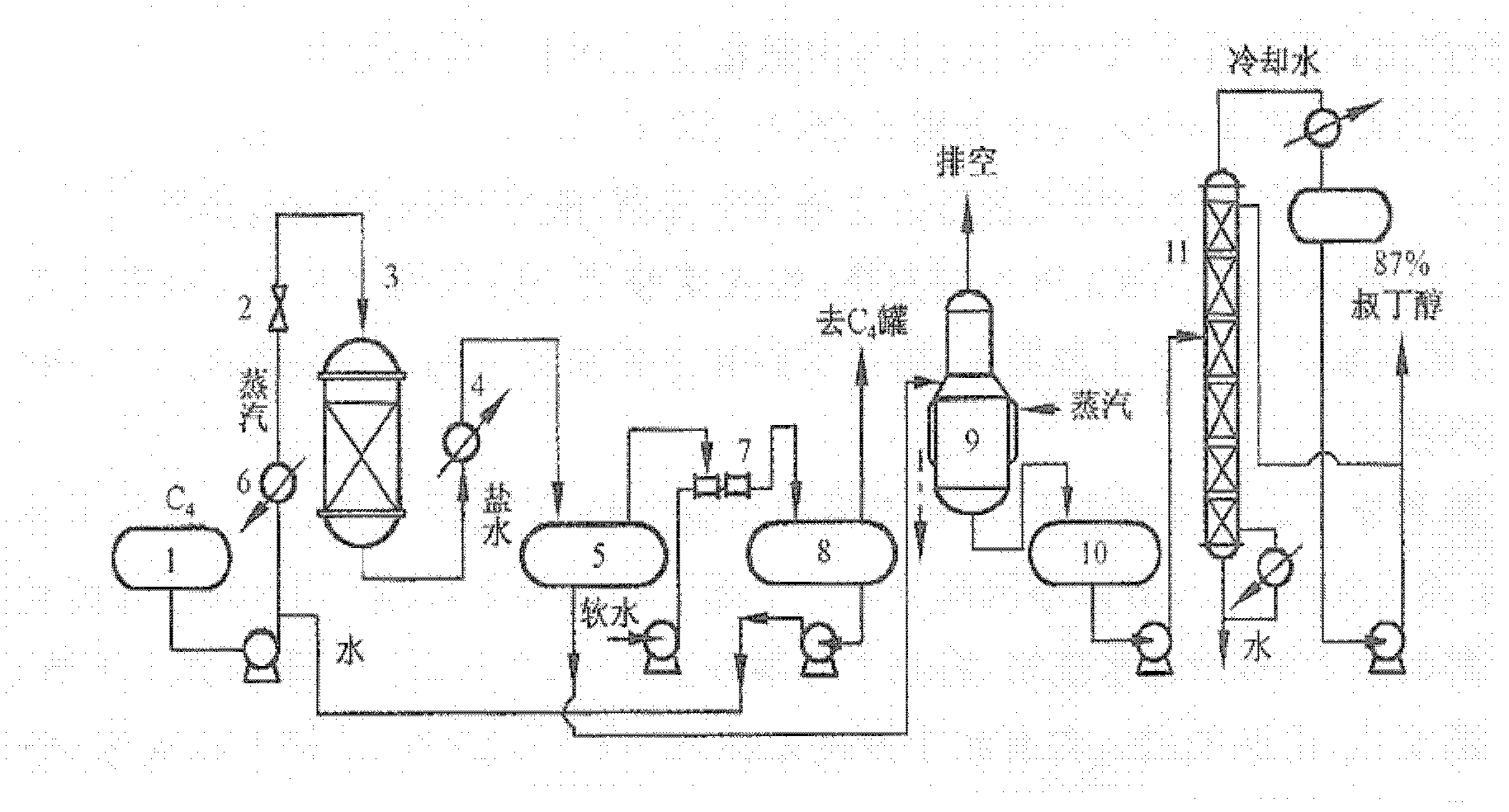
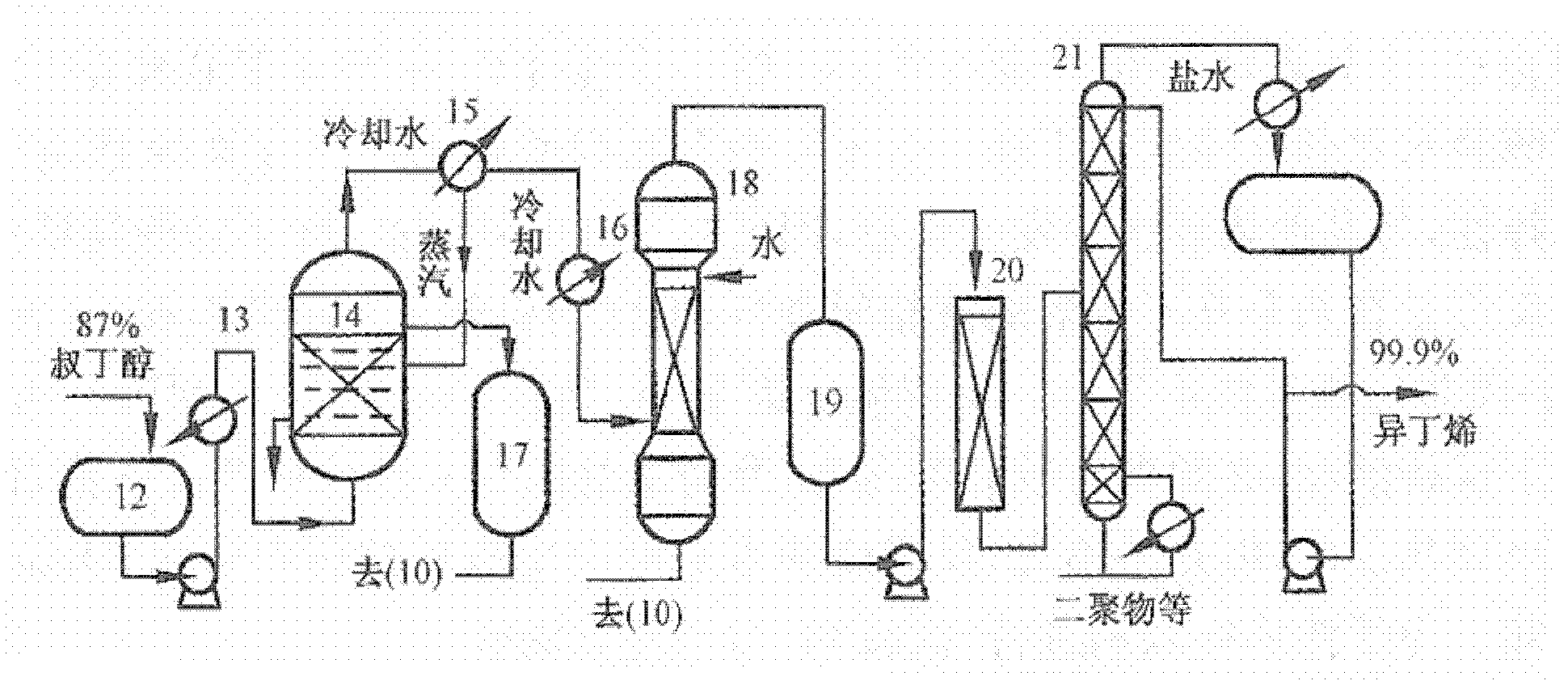
Method for preparing isobutene through direct hydration method
A technology of isobutene and hydration method, which is applied in the direction of hydrocarbon production from oxygen-containing organic compounds, chemical transformation purification/separation, etc., and can solve problems such as high energy consumption, high reaction temperature, and single product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In the C4 fraction, the isobutene separation method using a strong acidic cation exchange resin as a catalyst separates isobutene from the mixed C4 in two steps. The first step is to hydrate the isobutene in mixed C4 to generate tert-butanol; the second step is to dehydrate tert-butanol to obtain high-purity isobutene. Due to the use of porous strongly acidic cation exchange resin as a catalyst, it has high selectivity and rarely reacts with other olefins, so the purity of tert-butanol is high, and the purity of isobutene is also high.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| critical temperature | aaaaa | aaaaa |
| critical pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com