Pharmaceutical composition composed of arsenic trioxide and Nandina domestica, its preparation method and its application
A technology of arsenic trioxide and Nantian bamboo, which is applied in the field of medicine, can solve the problems of restricting the application of this kind of medicine and unsafety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] The acute toxicity comparison test of the pharmaceutical composition and the original drug of arsenic trioxide:
[0012] The improved Cole's method was used to carry out the oral acute toxicity comparison test in mice, and the results showed (see Table 1): the LD50 of the pharmaceutical composition was greater than the LD50 of the original drug of arsenic trioxide, and the growth status of the animals was relatively better.
[0013] Table 1. Results of oral acute toxicity comparison test in mice
[0014]
Embodiment 2
[0016] In vitro anti-tumor activity comparison test between the pharmaceutical composition and the original drug of arsenic trioxide:
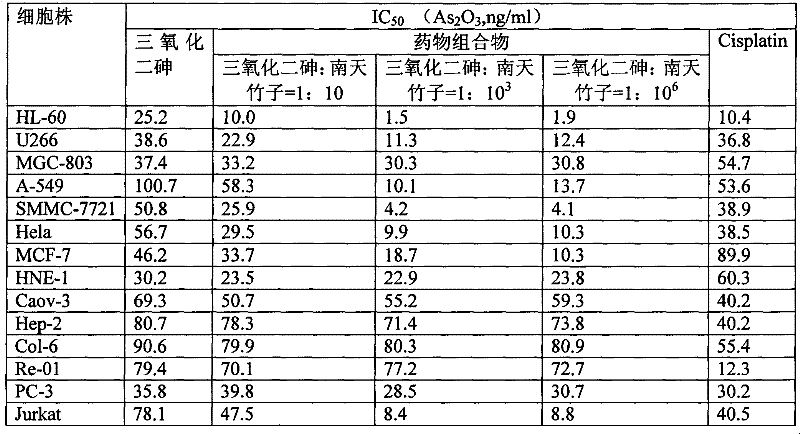
[0017] Adopt the MTT method to measure the inhibitory effect of arsenic trioxide original drug and pharmaceutical composition on the growth of the following human tumor cells in vitro, and measure the half maximal killing concentration (IC 50 ), the positive control drug was cisplatin (Cisplatin).
[0018] Human tumor cell lines: promyelocytic leukemia cell line HL-60, multiple myeloma cell line U266, gastric cancer cell line MGC-803, lung cancer cell line A-549, liver cancer cell line SMMC-7721, cervical cancer cell line Hela, Breast cancer cell line MCF-7, nasopharyngeal cancer cell line HNE-1, ovarian cancer cell line Caov-3, laryngeal cancer cell line Hep-2, colon cancer cell line Col-6, kidney cancer cell line Re-01, prostate cancer cell line Cancer cell line PC-3, T cell lymphoma cell line Jurkat.
[0019] The test results show (see Ta...
Embodiment 3
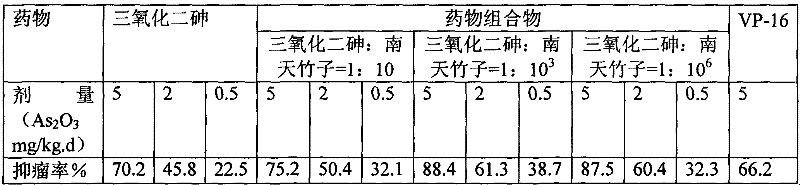
[0023] Comparison of the anti-tumor activity of the pharmaceutical composition and the original drug of arsenic trioxide in vivo:
[0024] The inhibitory effect of the original arsenic trioxide drug and the pharmaceutical composition on the growth of S180 sarcoma in mice was compared, and the tumor inhibition rate was determined.
[0025] Kunming mice, half male and half female, weighing 20g±2g, were subcutaneously inoculated with S180 mouse tumor cells 4×10 in the right armpit of each mouse 6 , to establish the S180 mouse tumor transplantation model. They were randomly divided into a normal saline group, a positive control group, and a high, medium and low dose administration group, with 10 rats in each group. 24 hours after inoculation, intragastric administration was started. The positive control group was given etoposide (VP-16) 4 mg / kg, and the treatment group was given high, medium and low doses of corresponding drugs, once a day for 10 consecutive days. The animals we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com