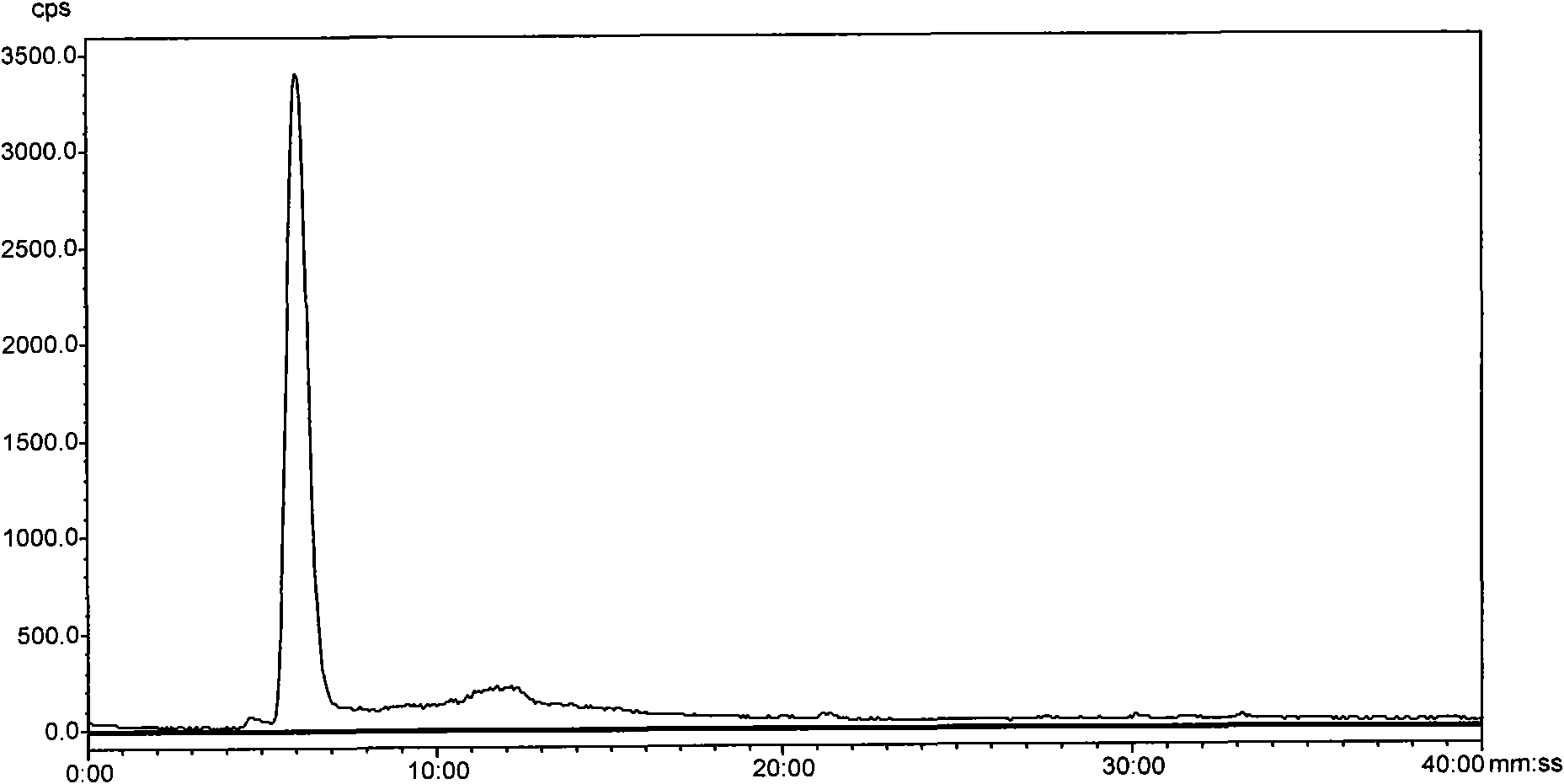
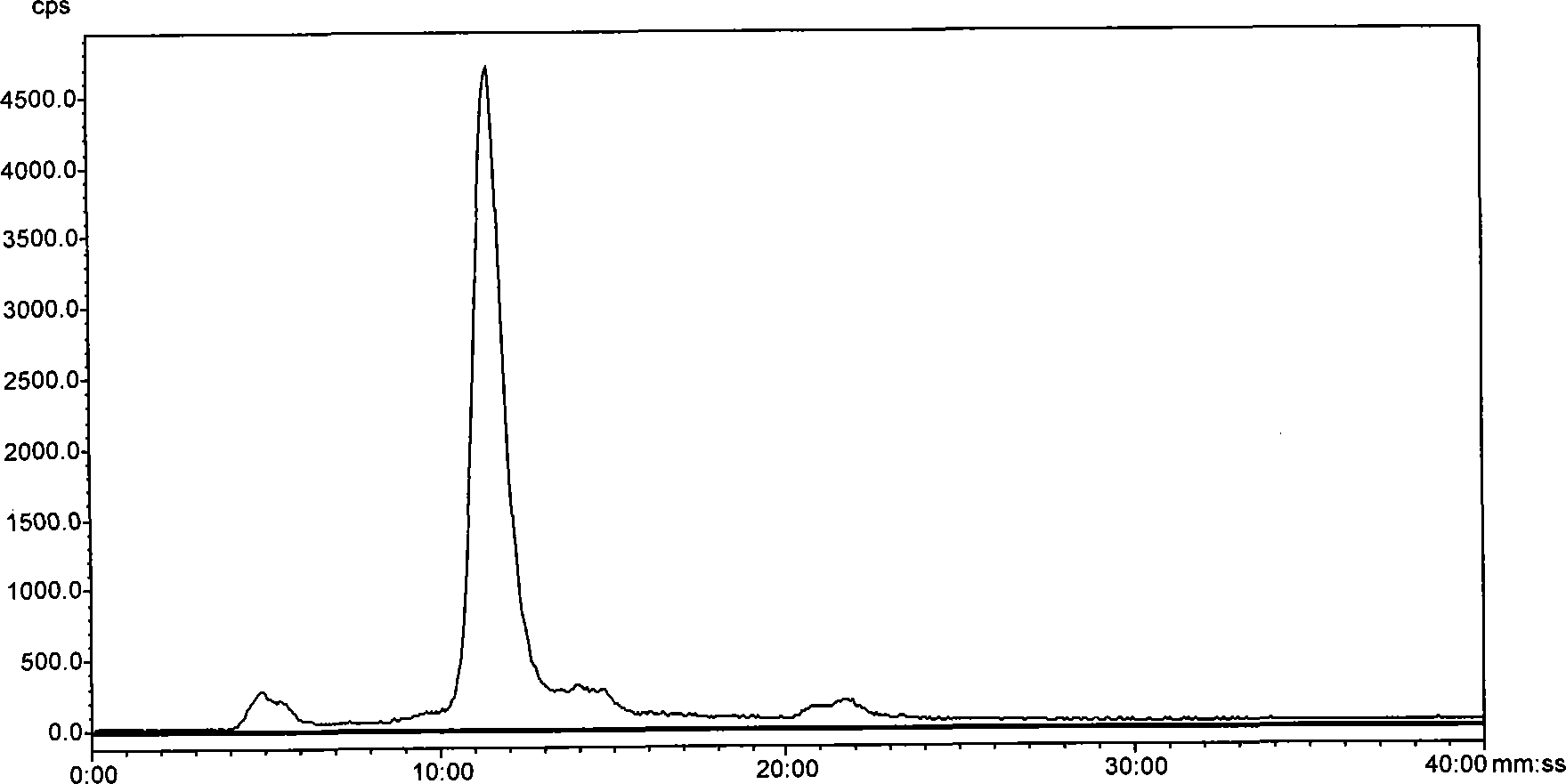
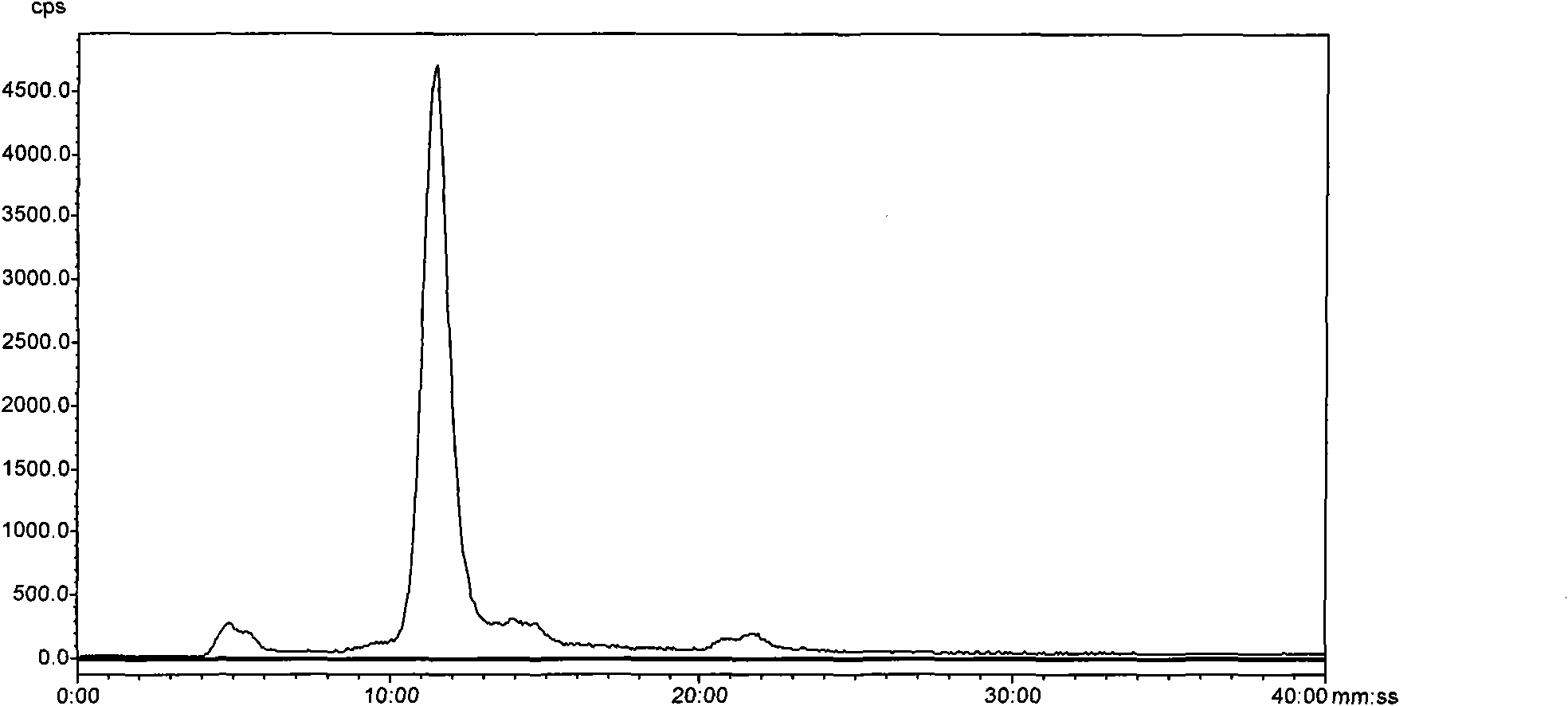
<99m>tc complex, and preparation method, intermediate and application thereof
A 99mtc, 99mtco4- technology, applied in the direction of radioactive carriers, can solve the problem of low labeling rate, achieve the effect of simple steps, excellent water solubility and stability, and overcome poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Synthesis of Compound V (n=81) (Partial Acetylation of Fifth Generation Polyamidoamine Dendrimer)
[0049]
[0050] According to the literature (Istva'n J.Majoros, Acetylation of Poly(amidoamine) Dendrimers, Macromolecules 2003, 36, 5526-5529) acetylation dendrimers, the specific steps are as follows: 100mg of the fifth generation polyamide-amine dendrimers ( Compound VI, G5 PAMAM, molecular weight 28824, stored in methanol, wt: 5%) (approximately 3.47×10 -6 mol), 24.9 μl of acetic anhydride (Ac 2 O) (about 2.43×10 -4 mol) and 43.4 μl of triethylamine (Et 3 N) (about 3.04×10 -4 mol) were mixed, stirred and reacted for 24 h at room temperature (25° C.) under nitrogen protection, and a colorless transparent liquid was obtained. Spin to dry the solvent, dissolve the product with deionized water, filter with a water-phase needle filter (specification 25mm×0.20μm), and dialyze the filtrate in PBS solution (PBS formula: NaCl 8.0g, KCl 0.2g, NaCl 2 HPO 4 1....
Embodiment 2
[0053] Example 2: Synthesis of Compound III (m=9, n=81) (Synthesis of PAMAM-Ac-Bt)
[0054]
[0055] 30mg biotin (1.24×10 -4 mol), 48mg EDC·HCl (2.48×10 -4 mol) and 36mgHOBt (2.48×10 -4 mol) was dissolved in 2ml DMSO, reacted at room temperature for 1h, then added 5ml dissolved in 100mg partially acetylated fifth-generation polyamido-amine dendrimers (G5-Ac, 3.1×10 -6 mol) in DMSO solution, reacted at room temperature for 1 d, diluted with deionized water, filtered to remove unreacted biotin, and the filtrate was dialyzed in PBS solution for one day, and the PBS solution was changed three times. The above product was continued to be dialyzed against deionized water for two days, changing the water three times a day. After dialysis, the obtained product was freeze-dried, and the yield was about 90.3%.
[0056] Its identification data are as follows:
[0057] 1 H-NMR (D 2 O, TMS, 500MHz), δ1.88 (244H, s), 2.33 (504H, br), 4.44 (9H, s), 4.62 (10H, s)
Embodiment 3
[0058] Example 3: Synthesis of Compound I (m=9, n=81, p=10) (Synthesis of PAMAM-Ac-Bt-DTPA)
[0059]
[0060] incl. 3 x 10 -6 mol G5-Ac-Bt aqueous solution (concentration 0.6mM), add 6×10 -5 mol of compound IV, adjust pH=9 with 1M NaOH solution, react at 40°C for 24 hours, dilute with deionization, filter with an aqueous needle filter (size 25mm×0.20μm), dialyze the filtrate in PBS solution for one day, replace PBS solution three times . The above product was continued to be dialyzed against deionized water for two days, changing the water three times a day. After dialysis, the obtained product was freeze-dried, and the yield was about 92.7%.
[0061] The NMR data are as follows:
[0062] 1 H-NMR (D 2 O, TMS, 500MHz), δ1.88(244H, s), 2.33(504H, br), 4.44(9H, s), 4.62(10H, s), 6.84(18H, m), 7.11(21H, m)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com