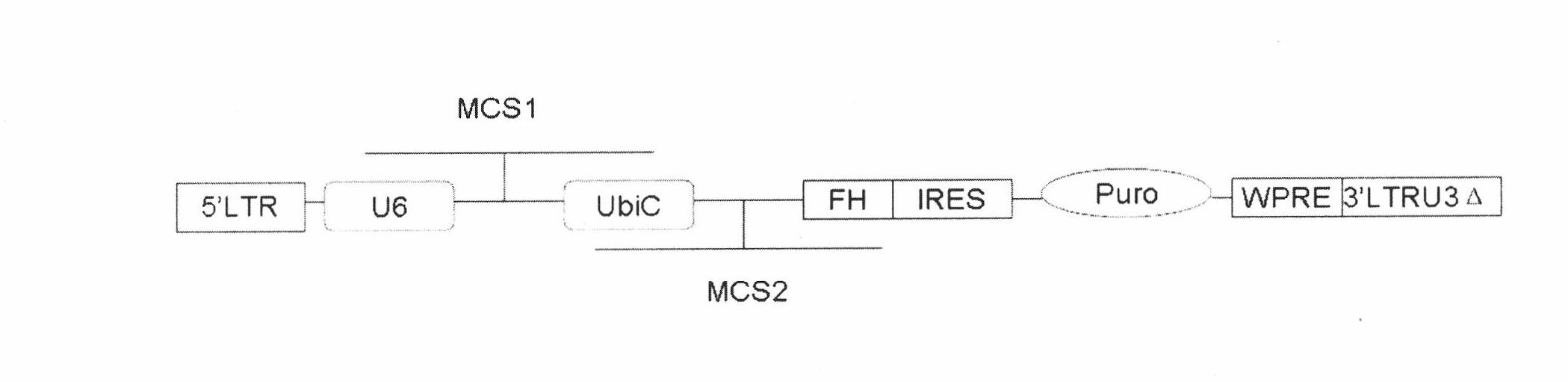
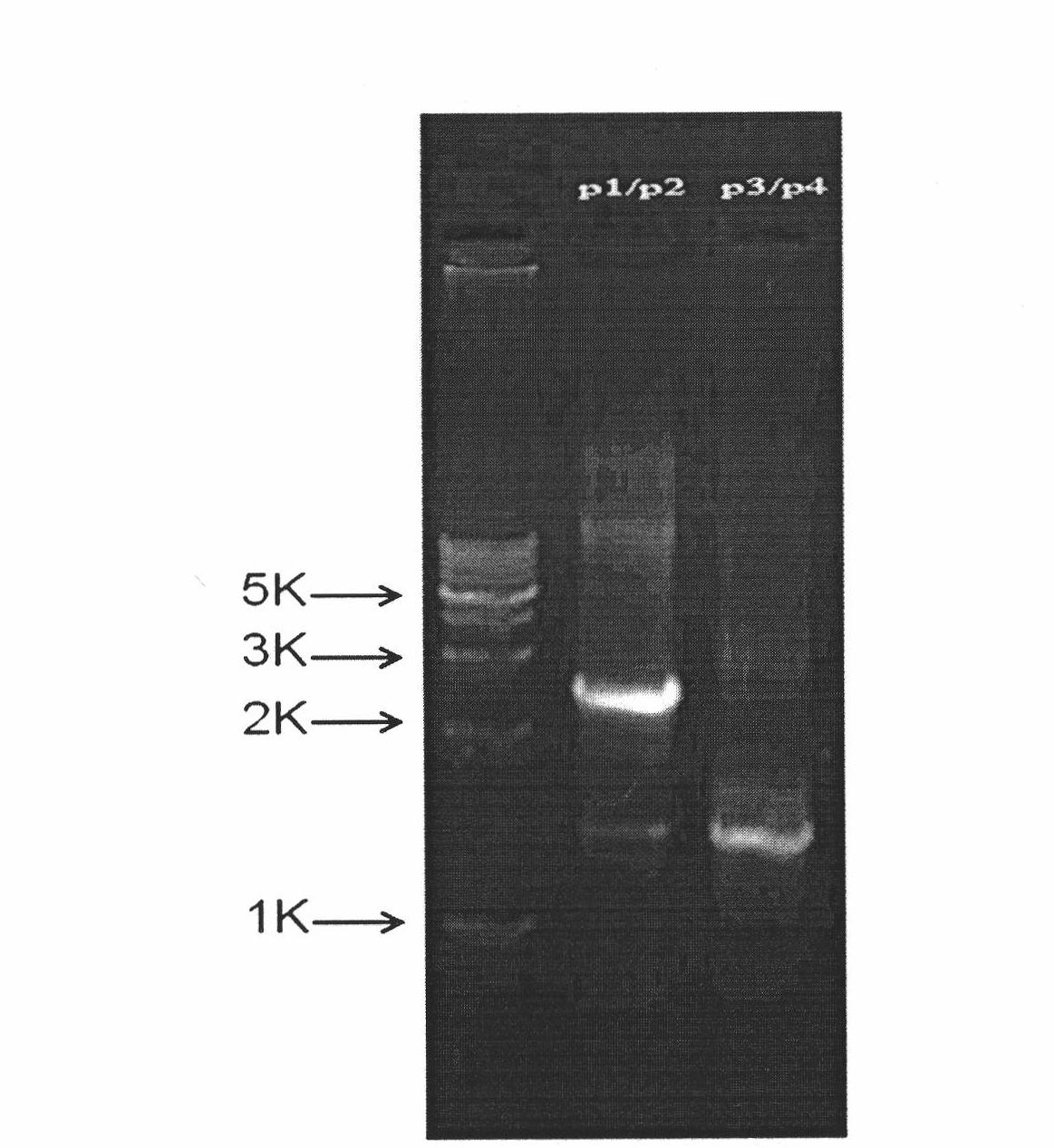
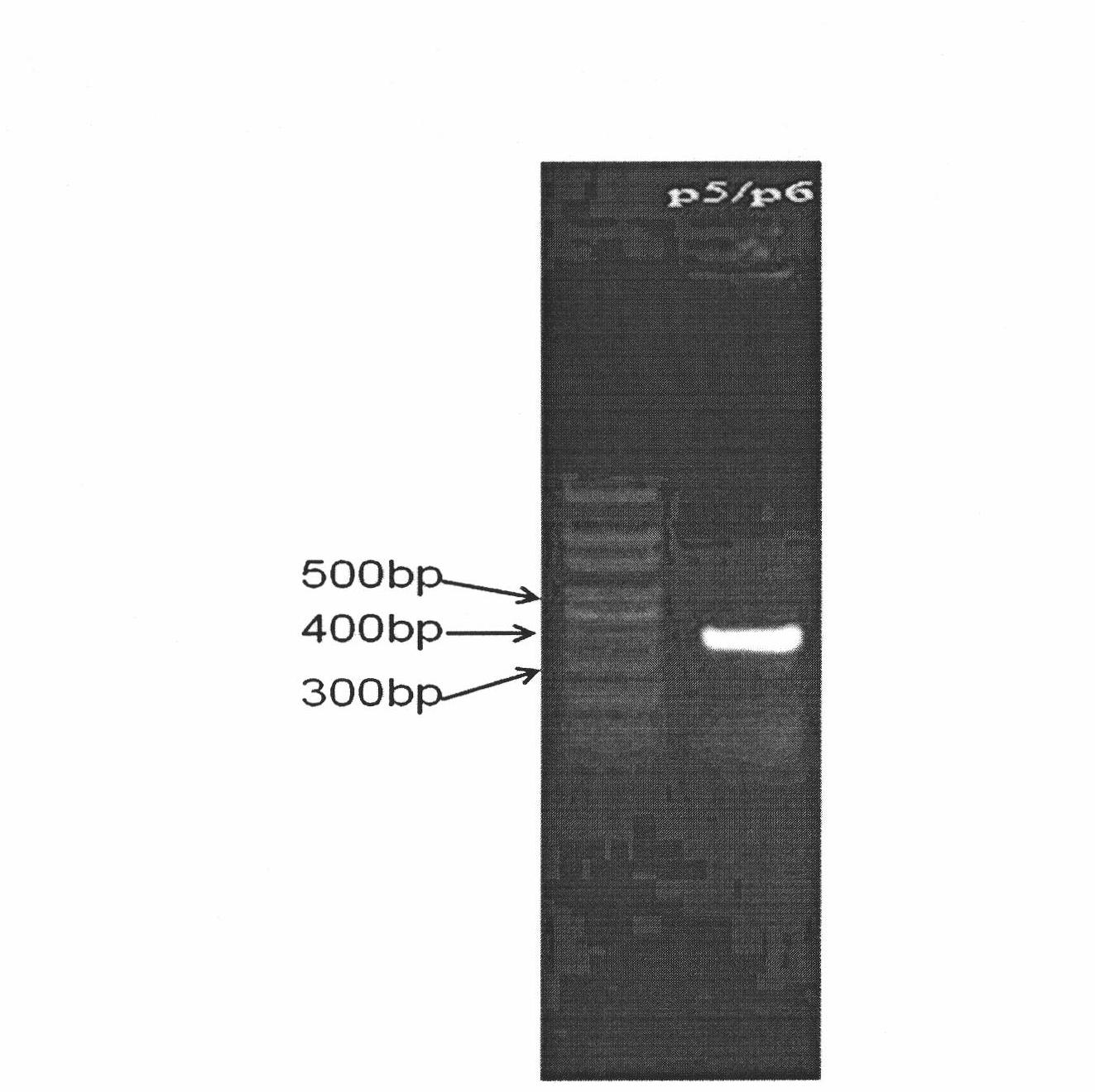
Multifunctional slow virus carrier capable of restraining endogenesis target genes and simultaneously expressing exogenous genes
A lentiviral vector and exogenous gene technology, applied in genetic engineering, plant genetic improvement, biochemical equipment and methods, etc., can solve problems such as off-target effects and siRNA difficulties, and achieve the effect of improving curative effect and eliminating negative effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Construction of α-ACTN1 shRNA expression cassette:
[0045] The predicted human α-ACTN1 shRNA target sequence is 5’GGGACACAGATCGAGAACATCGAAGAG( Figure 7 ). Design a pair of complementary oligonucleotides with the following sequences:
[0046] A1:
[0047] 5’-CTAGAGGGACACAGATCGAGAACATCGAAGAGTTCAAGAGACTCTTCGA TGTTCTCGATCTGTGTCCCTTTTTC
[0048] A2:
[0049] 5'TCGAGAAAAAGGGACACAGATCGAGAACATCGAAGAGTCTCTTGAACTCTTCGATGTTCTCGATCTGTGTCCCT
[0050] Mix the pair of primers A1 / A2 in equal amounts, denature at 95°C for 5 minutes, and cool and anneal naturally. Ligated with the lentiviral vector PU2-FH digested with XbaI / XhoI to obtain the plasmid PU2-α-ACTN1shRNA.
Embodiment 2
[0051] Embodiment 2: Construction of anti-RNAi human α-ACTN1 (rr-α-ACTN1) cDNA ( Figure 7 )
[0052] According to the siRNA target sequence, without affecting the amino acid sequence, design primers containing 4 point mutations, the sequence is as follows:
[0053] (1); 5'-AGAGAATTCCATGGACCATTATGATTCTCAGCAAAC,
[0054] (2); 5'-CCTCTTCAATATTTTCAATCTGTGTCCCCGCCTTCCGGAG,
[0055] (3); 5'-CACAGATTGAAAATATTGAAGAGGACTTCCGGGATGGCCTG,
[0056] (4); 5'-CTCGGTACCGGTGAGGTCACTCTCGCCGTACAG,
[0057] Using the plasmid GC-Z6382 (purchased from genecopoeia) as a template, the N-terminal fragment (N-ter) of rr-α-ACTN1 was amplified with 1 and 2 primers, and the C-terminal fragment (C-ter) was amplified with 3 and 4 primers Amplify, and then use ligation PCR to use 1, 4 as primers, and two purified mixed PCR product fragments as templates to amplify the full-length rr-α-ACTN1, which is digested with EcoRI / AgeI and cloned into the same EcoRI / AgeI In the lentiviral plasmid PU2-α-ACTN1 shRNA...
Embodiment 3
[0061] Example 3: Production and introduction of lentivirus
[0062] 293T cells (purchased from ATCC) were cultured in a petri dish with a diameter of 10 cm, and the packaging plasmid pHR'8.2deltaR / pCMV-VSV-G (purchased from addgene) was used in a ratio of 8:1 (the total DNA amount was 5 μg) respectively. Co-transfect with two lentiviral recombinant plasmids: PU2-α-ACTN1 shRNA, PU2-α-ACTN1 shRNA+rr-α-ACTN1+GFP (5 μg each), replace the culture medium after 24 hours, and start from transfection the next day The virus-containing culture fluid was collected from the 293T cells and filtered through a 0.45 μm filter to remove residual 293T cells. When infecting Jurkat T cells (purchased from ATCC), mix the virus with an equal volume of fresh medium, add protamine sulfate (final concentration of 10 μg / ml, Sigma), and culture the cells for 6 hours or overnight, then replace with fresh medium without After 24 hours, puromycin was added to the virus culture solution for selection until...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com