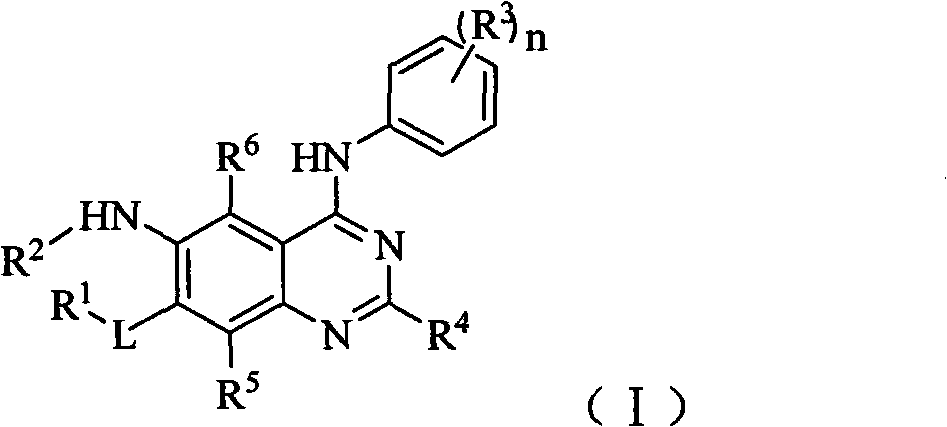
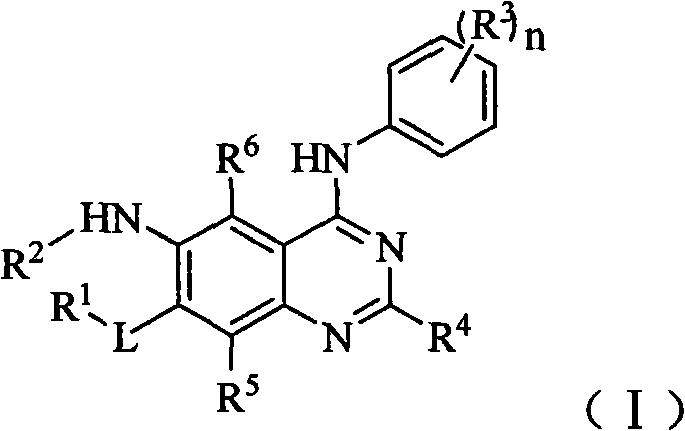
Aniline substituted quinazoline derivative
A technology of substituents and amine groups, which is used in the field of preparing drugs for the treatment of tumors, can solve the problems of uncontrolled cell reproduction, abnormal mutation or overexpression, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
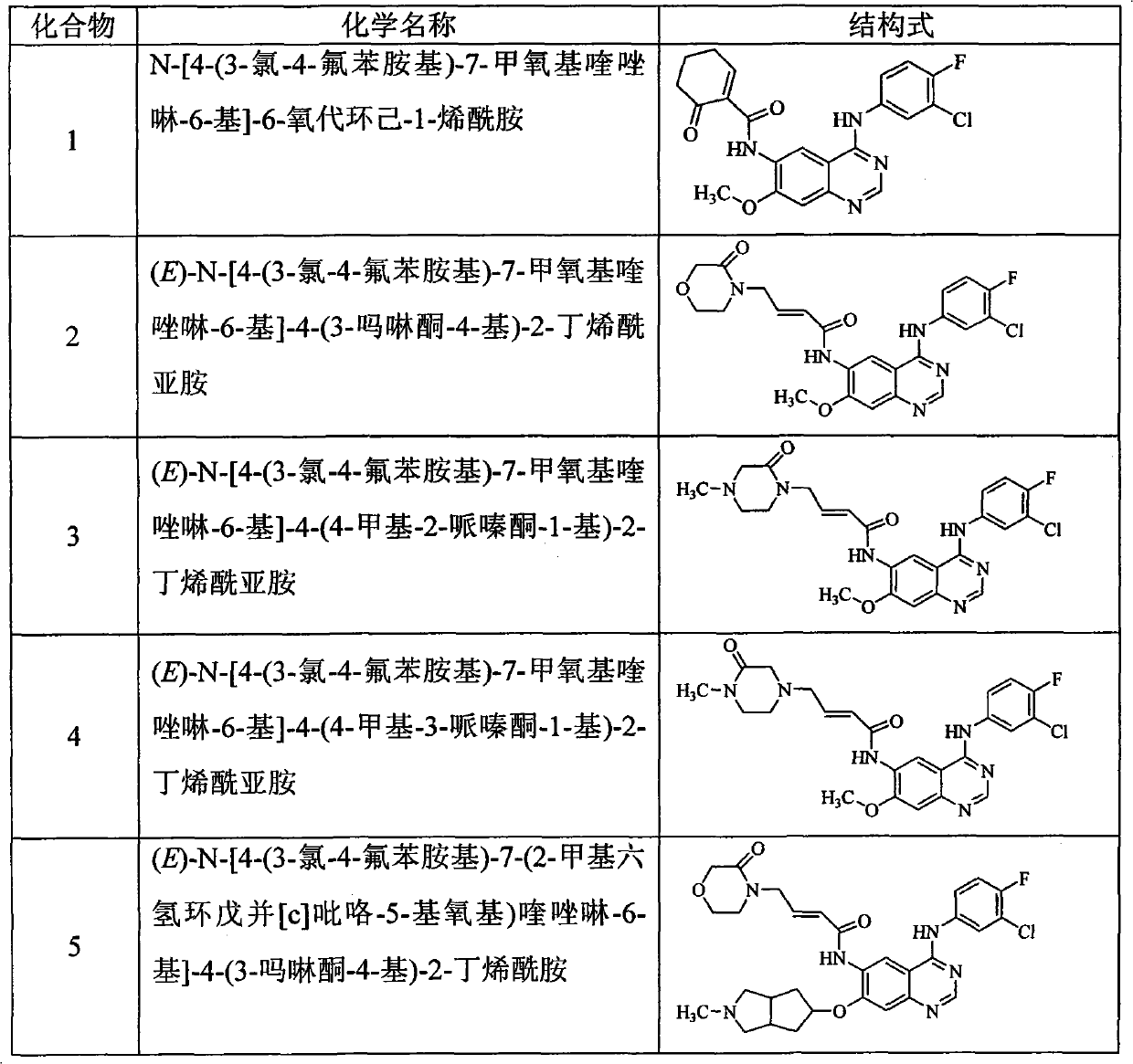
[0197] Example 1N-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl]-6-oxocyclohex-1-enamide (compound 1) preparation
[0198]
[0199] (1) Preparation of N-(4-(3-chloro-4-fluoroaniline)-7-methoxyquinazolin-6-yl)-2-oxocyclohexylamide
[0200]
[0201] 2-oxocyclohexanoic acid (670mg, 4.72mmol), HATU (2.69g, 7.08mmol) and DIEA (1.22g, 9.46mmol) were dissolved in 20mL DMF, stirred at room temperature for 20min, then added N 4 -(3-Chloro-4-fluoroaniline)-7-methoxyquinazoline-4,6-diamine (1.0g, 3.14mmol), reacted at 50°C for 24h. Add water, extract with dichloromethane, dry, concentrate and separate by column chromatography (dichloromethane:methanol=30:1) to obtain N-(4-(3-chloro-4-fluoroaniline)-7-methoxyquin Azolin-6-yl)-2-oxocyclohexylamide 570 mg, yield 41%.
[0202] (2) Preparation of N-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl]-6-oxocyclohex-1-enamide
[0203]
[0204] Under an ice-water bath, dissolve pyridine (102mg, 1.29mmol) and phenylsel...
Embodiment 2
[0207] Example 2 (E)-N-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl]-4-(3-morpholinone-4-yl )-2-butenamide (C Compound 2) Preparation
[0208]
[0209] (1) Preparation of (E)-4-(3-morpholinone-4-yl)butenoic acid methyl ester
[0210]
[0211] 3-Morpholinone (10.1g, 0.1mol), methyl 4-bromocrotonate (25g, 0.12mol), cesium carbonate (97g, 0.3mol) and PdCl 2 (dppf)(2g, 2.7mmol) was dissolved in 200mL of dioxane, heated to 100°C for 12h. Add water and extract with ethyl acetate, dry, concentrate and separate by silica gel column to obtain 5.2 g of oily substance with a yield of 41%.
[0212] (2) Preparation of (E)-4-(3-morpholinone-4-yl)butenoic acid
[0213]
[0214] Dissolve (E)-4-(3-morpholinone-4-yl)butenoic acid methyl ester (500mg, 2.5mmol) in 30mL methanol, add barium hydroxide (476mg, 1.5mmol) at -10°C Aqueous solution, stirred and reacted for 16h. After the reaction, the pH was adjusted to 2, extracted with ethyl acetate, dried, concentrated and...
Embodiment 3
[0220] Example 3 (E)-N-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl 1-4-(4-methyl-2-piperazine Keto-1-yl)-2-butene Preparation of Amide (Compound 3)
[0221]
[0222] (1) Preparation of 4-methyl-2-piperazinone
[0223]
[0224]Dissolve 2-piperazinone (2.0g, 20mmol) and 5mL aqueous formaldehyde solution in 20mL THF, add sodium borohydride (2.28g, 60mmol) under ice water, rise to room temperature and react for 2h, then quench with pasty sodium sulfate , filtered, and the solvent was evaporated to dryness to obtain 4-methyl-2-piperazinone. The product was directly used in the next reaction without further treatment.
[0225] (2) Preparation of (E)-4-(4-methyl-2-piperazinone-1-yl)butenoic acid methyl ester
[0226]
[0227] 4-Methyl-2-piperazinone (1.5 g, 13 mmol) was added to a THF solution of NaH (60%, 720 mg, 18.0 mmol) under an ice-water bath, and reacted for 20 min. Methyl 4-bromocrotonate (2.31 g, 13 mmol) was added dropwise and the reaction was co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com