Preparation method of acetylated epigallocatechin gallate
A technology of epigallocatechin and gallate, applied in the field of preparing acetylated epigallocatechin gallate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
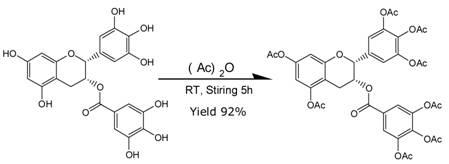
[0025] Add 9.2g (about 0.02mol) of EGCG to a 250ml three-neck flask with a magnet, dissolve it with 40-200ml of ethyl acetate, preferably 60-100ml of ethyl acetate as a solvent, add 20-160ml of acetic anhydride (about 0.1 ~0.8mol), the best dosage is 30-60ml.
[0026] Add catalyst ZnCl 2 1.36~27.6g (about 0.01~0.2mol), preferably 6.8~20.4g (0.05~0.15mol) as the catalyst. Stir at room temperature for 3-8 hours. As the reaction continued, the homogeneity of the system continued to increase, and the system gradually changed from a yellow turbid liquid to a transparent light brown liquid, and the reaction was detected by TLC. Add 80~200ml of saturated sodium bicarbonate, continue to stir for 1 h, separate the ethyl acetate layer, then extract the water phase with ethyl acetate (100ml / time) for 3 times, combine and dry the ethyl ester layer, and remove the ethyl acetate by rotary evaporation. 16g of light brown transparent oil was obtained, with a yield of 98%. Recrystallized onc...
Embodiment 2
[0028] Add 9.2g (about 0.02mol) of EGCG to a 250ml three-neck flask with a magnet, dissolve it with 40-200ml of ethyl acetate, preferably 60-100ml of ethyl acetate as a solvent, add 20-160ml of acetic anhydride (about 0.1 ~0.8mol), the best dosage is 30-60ml.
[0029] Add catalyst ZnAc 24.25~37g (about 0.025~0.2 mol) The best dosage can be 13.88~24 (about 0.075~0.12mol). Stirred at 80 DEG C for 5h, along with the continuous progress of the reaction, the uniformity of the system was continuously enhanced, and the system gradually changed from a yellow turbid liquid to a transparent dark brown liquid, and the TLC detection reaction ended. Add 80ml of saturated sodium bicarbonate, continue to stir for 1 h, separate the ethyl acetate layer, then extract the water phase with ethyl acetate (100ml / time) for 3 times, combine and dry the ethyl ester layers, remove the ethyl ester by rotary evaporation, and obtain 15.7 g Dark brown transparent oil, yield 96%, recrystallized once with ...
Embodiment 3
[0031] Add 1840g (about 4mol) of EGCG into a 50L reactor equipped with mechanical stirring, dissolve it with 8~36L of ethyl acetate, the optimum solvent volume is 12~24L, add 6-16L of acetic anhydride (about 30~80mol), and add the catalyst ZnCl 2 170~1360g (about 1.25~10mol), the best dosage is 510~1020g (about 3.65~7.5mol), and the system is a heterogeneous system. Stirring at room temperature for 7 hours, as the reaction continued to increase the uniformity of the system, the system gradually changed from a yellow turbid liquid to a transparent light yellowish brown liquid, and TLC detected that the reaction was over. Add 16L of saturated sodium bicarbonate, continue to stir for 1 h, separate the ethyl acetate layer, and then extract the water phase with ethyl acetate (20L / time) in the extraction tower for 3 times, combine and dry the ethyl layer, and remove the ethyl acetate layer by rotary evaporation. Esters, about 3100g light brown transparent oil was obtained, with a y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com