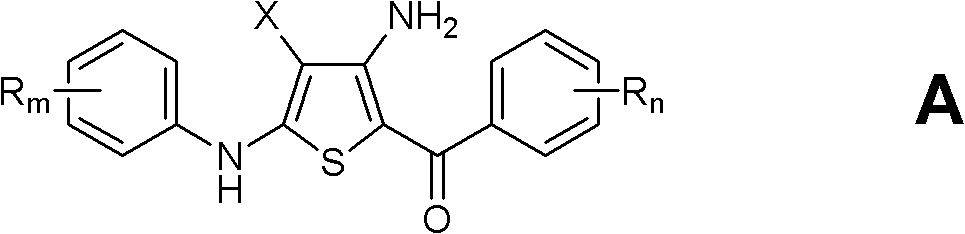
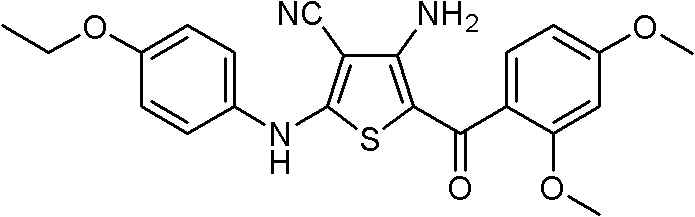
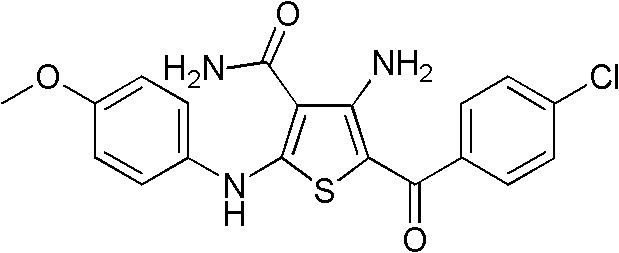
Substituted 4-amino-5-benzoyl-2-(phenylamino)thiophene-3-carbonitriles and substituted 4-amino-5-benzoyl-2-(phenylamino)thiophene-3-carboxamides as tubulin polymerization inhibitors
A technology of polymerization inhibitor and tubulin, which can be used in drug combinations, medical preparations containing active ingredients, organic chemistry, etc., and can solve problems such as no activity being given.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0081] The following examples illustrate the preparation of compounds of the invention and their activity in predictive in vitro and in vivo anticancer assays. These results are believed to be predictive for efficacy in human anticancer chemotherapy, as other anticancer agents tested in these assays have shown anticancer activity in humans. They are given to enable those skilled in the art to understand and realize the present invention more clearly, but these embodiments should not be considered as limiting the scope of the present invention, but merely to illustrate and describe the present invention.
[0082] Preparation and Synthesis Examples
[0083] The compounds of the present invention can be prepared by conventional methods of organic chemistry. See, eg, Larock, "Comprehensive Organic Transformations", Wiley-VCH, New York, New York, U.S.A. In some cases, protecting groups can be introduced and subsequently removed. Suitable protecting groups are described in Greene...
preparation Embodiment 1
[0084] Preparation Example 1. Preparation of 4-[2-(dimethylamino)ethoxy]phenylisothiocyanate, which is an intermediate of compound 36A
[0085]Diisopropylethylamine (344mg, 2.66mmol, 2.4eq. (eq.)) was added to 4-[2-(dimethylamino)ethoxy]aniline (200mg, 1.11mmol, 1eq.) with stirring In a solution (0° C.) of tetrahydrofuran (2 mL), thiophosgene (153 mg, 1.33 mmol, 1.2 eq.) was then added dropwise with stirring. The reaction mixture was allowed to warm to room temperature with stirring and stirred at room temperature for 40 minutes during which time a precipitate formed. THF was removed in vacuo and the residue was dissolved in ethyl acetate, washed with brine (3 x 10 mL), then the ethyl acetate layer was dried over magnesium sulfate and the ethyl acetate was evaporated to give 4-[2-( Methylamino)ethoxy]phenylisothiocyanate (0.20 g) as a tan oil was used without further purification.
preparation Embodiment 2
[0086] Preparation Example 2: Preparation of 2-bromo-4'-[2-(dimethylamino)ethoxy]acetophenone, which is an intermediate of compound 39A
[0087] 2-(Dimethylamino)ethyl chloride hydrochloride (3.49g, 23.2mmol, 1.1eq.) was added to 4'-hydroxyacetophenone (3.00g, 22.0mmol, 1eq.) in acetone (100mL) To the solution in , potassium carbonate (9.12 g, 66.0 mmol, 3 eq.) was then added. The reaction mixture was heated at reflux overnight, cooled, and filtered; the filtrate was concentrated, and water (50 mL) was added, forming a cloudy solution. Hydrochloric acid (0.1 N, 20 mL) was added and the resulting clear solution was extracted three times with ethyl acetate. The aqueous phase was made basic with aqueous sodium hydroxide to form a turbid mixture which was extracted with ethyl acetate (5 x 50 mL), then the ethyl acetate layer was dried over magnesium sulfate and the ethyl acetate was evaporated to give 4'-[2 - (Dimethylamino)ethoxy]acetophenone (2.90 g) as pale yellow oil, identi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com