New synthesis process for 2,4-bis(2-chloro-4-trifluoromethylphenoxy-nitrobenzene
A technology of trifluoromethylphenoxy and trifluoromethylphenol, which is applied in the field of new synthesis technology of 2,4-bis(2-chloro-4-trifluoromethylphenoxy)-nitrobenzene, Achieve the effect of reducing the amount of three wastes, easy industrialization, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
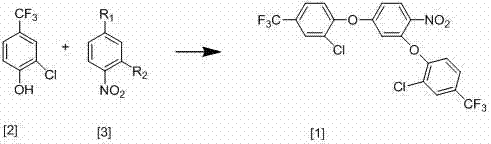
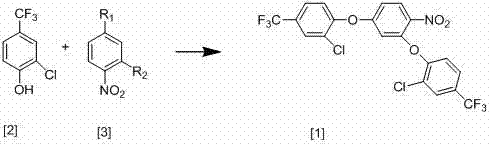
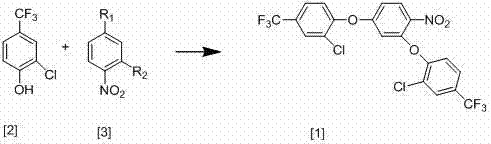
[0021] The new synthesis process of 2,4-bis(2-chloro-4-trifluoromethylphenoxy)-nitrobenzene of the present invention, the synthesis process is shown in the following equation:
[0022]
[0023] where: R 1 represents Cl;
[0024] R 2 represents Cl;
[0025] React 86g of 2-chloro-4-trifluoromethylphenol with 40g of 3,4-dinitrochlorobenzene, add 200ml of anhydrous DMF, 67g of anhydrous potassium carbonate and 1.6g of copper powder and stir at 80°C for 15 hours until the reaction Complete, after the reaction is over, cool the reaction mixture to room temperature, then filter, distill the filtrate under reduced pressure to recover DMF, then add 300ml of water and 300ml of toluene, stir well, let stand to separate layers, extract the water layer with toluene, and combine the toluene layers , the toluene layer contains 83g 2,4-bis(2-chloro-4-trifluoromethylphenoxy)-nitrobenzene, and the yield is 80%;
[0026] After the reaction, 2,4-bis(2-chloro-4-trifluoromethylphenoxy)-nitro...
Embodiment 2
[0029] The new synthesis process of 2,4-bis(2-chloro-4-trifluoromethylphenoxy)-nitrobenzene of the present invention, the synthesis process is shown in the following equation:
[0030]
[0031] where: R 1 stands for F;
[0032] R 2 stands for F;
[0033] React 86g of 2-chloro-4-trifluoromethylphenol with 32g of 3,4-dinitrofluorobenzene, add 200ml of anhydrous DMF, 67g of anhydrous sodium ethylate and 1.7g of copper powder and stir at 82°C for 18 hours until the reaction is complete , after the reaction, the reaction mixture was cooled to room temperature, then filtered, the filtrate was distilled under reduced pressure to reclaim DMF, then added 300ml of water and 300ml of toluene, fully stirred, left to stand for layering, the water layer was extracted with toluene, and the toluene layers were combined. The toluene layer contained 85 g of 2,4-bis(2-chloro-4-trifluoromethylphenoxy)-nitrobenzene, and the yield was 85%.
[0034] The invention has the advantages of mild re...
Embodiment 3
[0036] The new synthesis process of 2,4-bis(2-chloro-4-trifluoromethylphenoxy)-nitrobenzene of the present invention, the synthesis process is shown in the following equation:
[0037]
[0038] where: R 1 represents Cl;
[0039] R 2 stands for NO 2 ;
[0040] React 86g of 2-chloro-4-trifluoromethylphenol with 31g of 2,4-dichloronitrobenzene, add 200ml of anhydrous DMF, 67g of anhydrous potassium carbonate and 1.8g of copper powder and stir at 88°C for 17 hours until the reaction Complete, after the reaction is over, cool the reaction mixture to room temperature, then filter, distill the filtrate under reduced pressure to recover DMF, then add 300ml of water and 300ml of toluene, stir well, let stand to separate layers, extract the water layer with toluene, and combine the toluene layers , the toluene layer contains 86g of 2,4-bis(2-chloro-4-trifluoromethylphenoxy)-nitrobenzene, and the yield is 88%;
[0041] After the reaction, 2,4-bis(2-chloro-4-trifluoromethylphenoxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com