Aminoglycoside compound and extracting separation method thereof
A compound and composition technology, applied in the field of chemical structure of aminoglycoside compounds and its extraction and separation, can solve difficult to purify and undiscovered problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
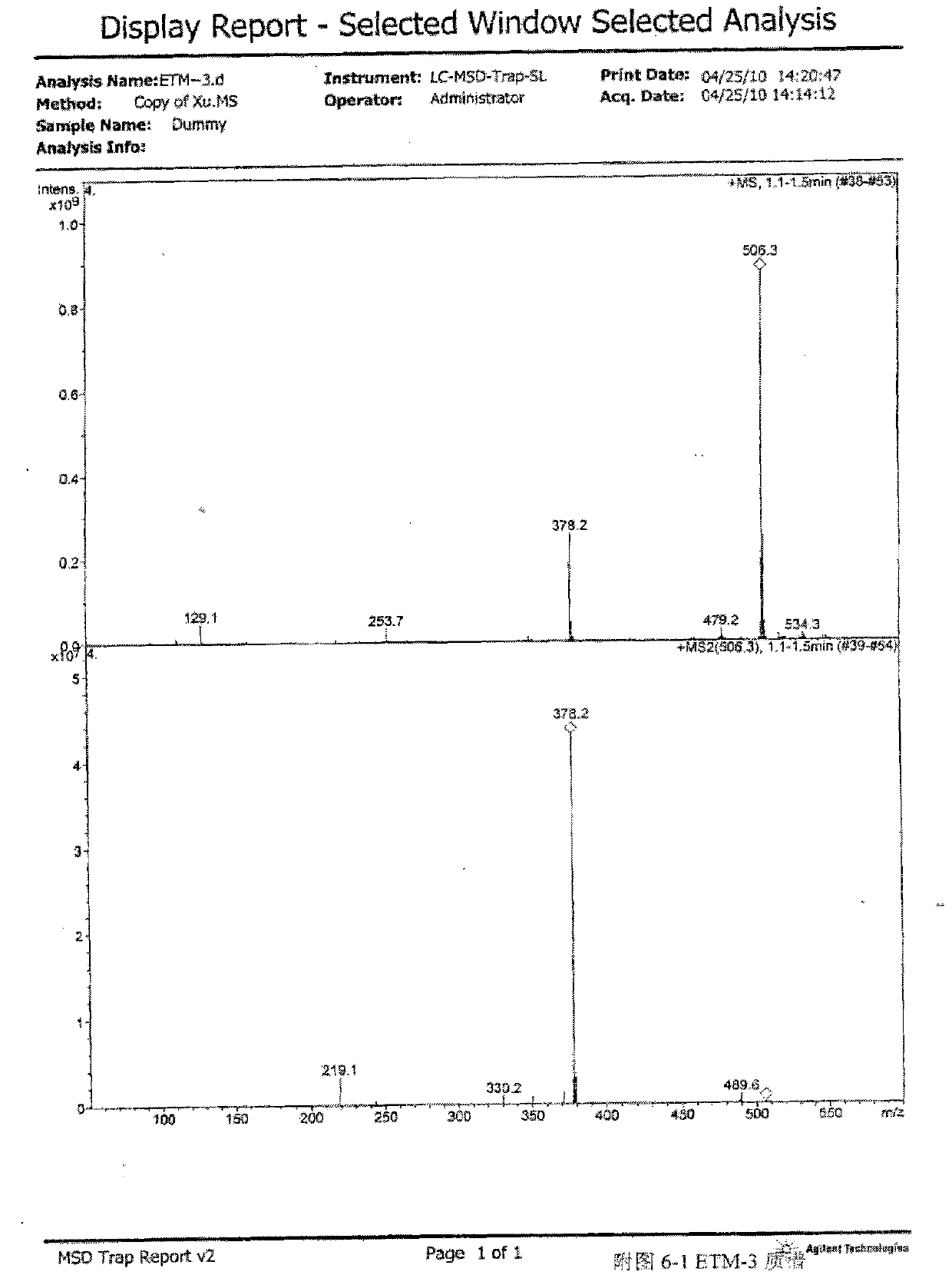
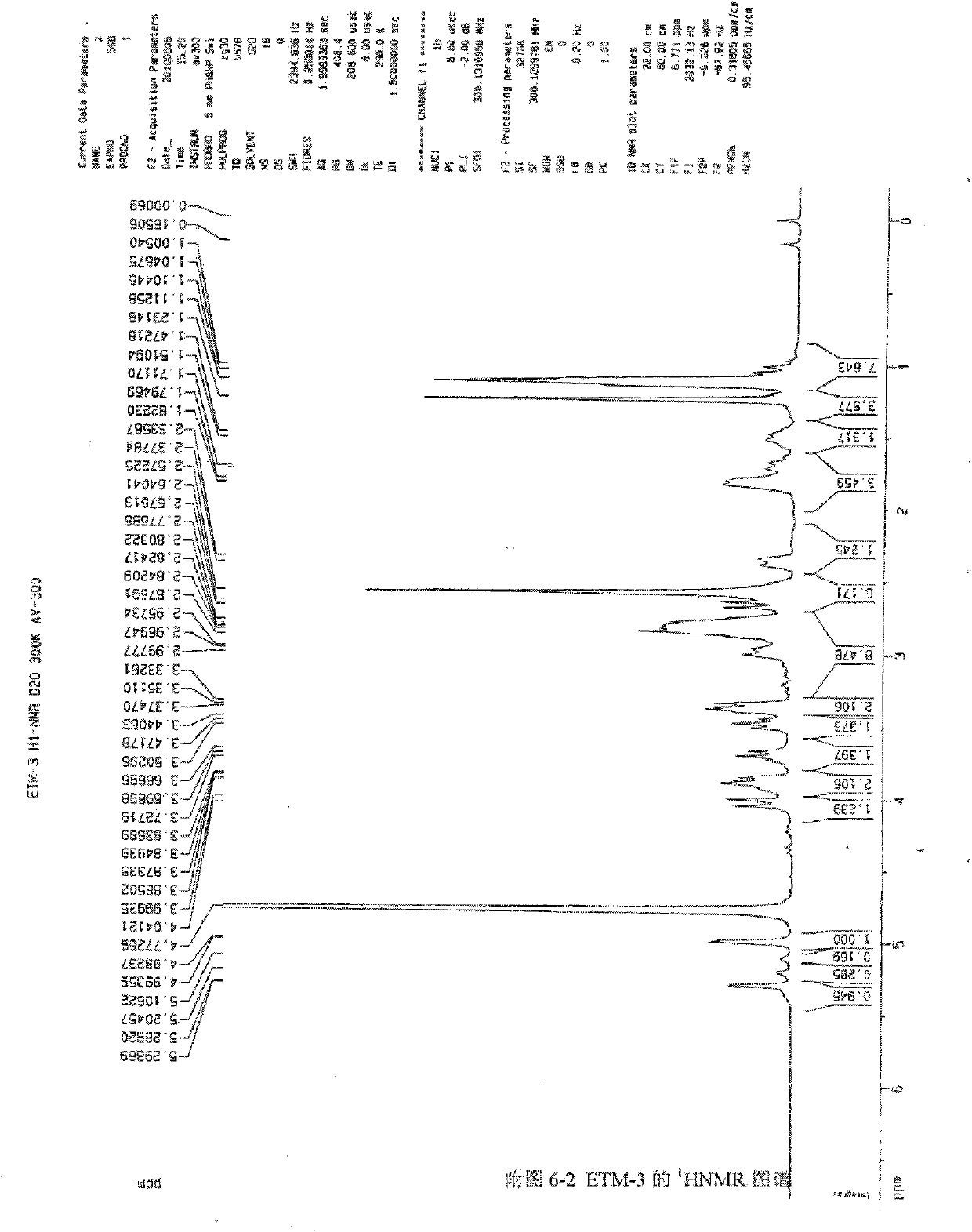
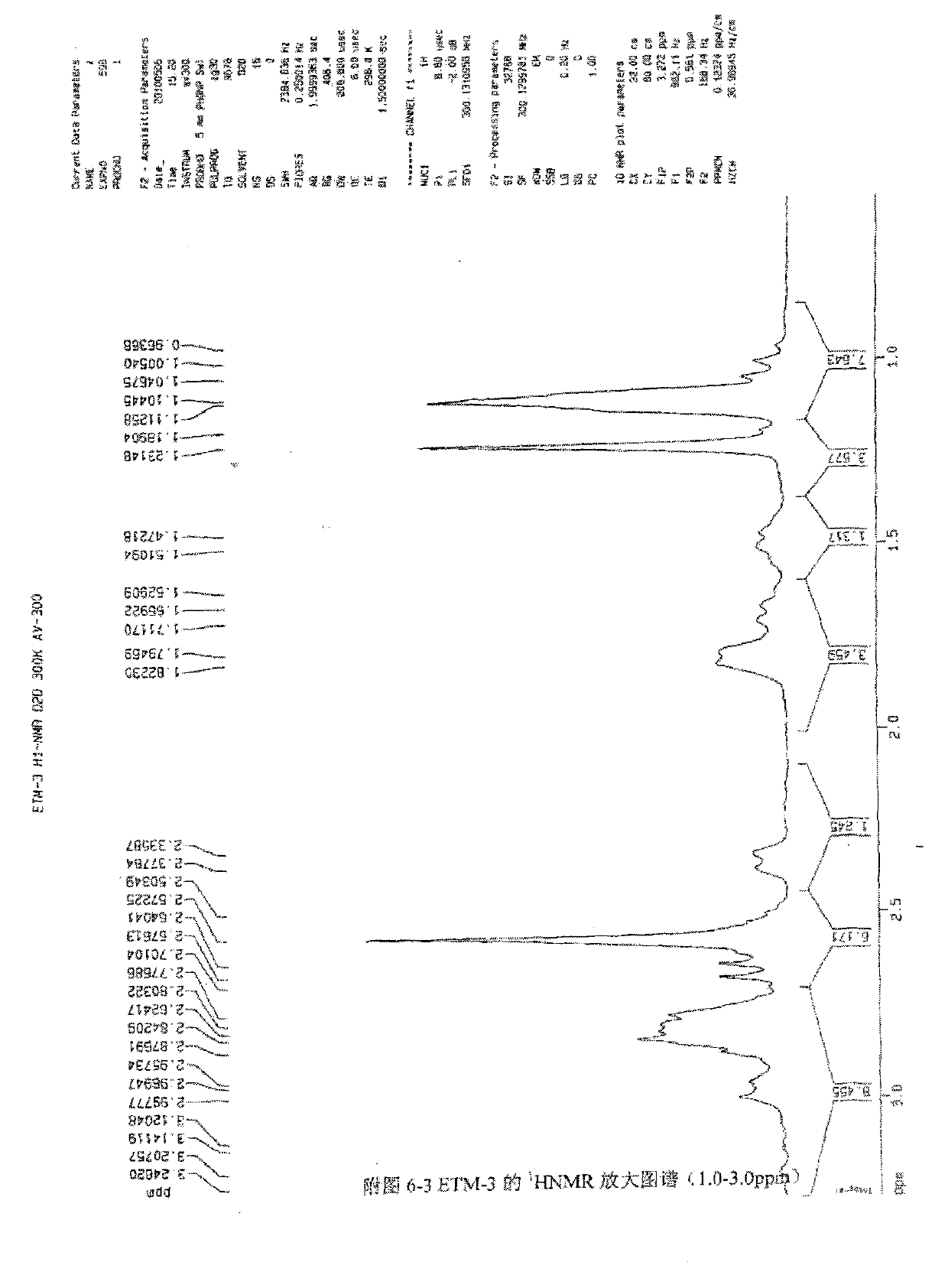
[0060] Get the etimicin sulfate crude product produced by the prior art, add water to dissolve, load the sample on HD-2 weakly acidic cation exchange resin column, use water respectively, ammoniacal liquor gradient elution, TLC detection (developing agent: chloroform-methanol-ammonia liquor , 1:1:1, lower layer, I2) and combined fractions to obtain impurity-enriched fractions. The impurity-enriched fractions were separated by column chromatography repeatedly using silica gel H (chloroform:methanol:ammonia water (2:1:1, lower layer) and CM-Sephadex C-25), and the sixth fraction was collected as Compound 1,3-N,N-diethylgentamycin C1a.
Embodiment 2
[0061] Embodiment 2, the preparation of hydrochloride:
[0062] Get the etimicin sulfate crude product produced by the prior art, add water to dissolve, load the sample on HD-2 weakly acidic cation exchange resin column, use water respectively, ammoniacal liquor gradient elution, TLC detection (developing agent: chloroform-methanol-ammonia liquor , 1:1:1, lower layer, I2) and combined fractions to obtain impurity-enriched fractions. The impurity-rich fractions were separated by column chromatography with silica gel H (chloroform:methanol:ammonia water (2:1:1, lower layer) and CM-Sephadex C-25 repeatedly, and the sixth fraction was collected and concentrated. to 200-500mg / ml, adjust the pH to 4.0-5.0 with 10mol / L hydrochloric acid, then add 2%-5% activated carbon, stir and decolorize at 60-80°C for 30 minutes, filter, and freeze-dry the filtrate to obtain the substance Hydrochloride solid.
Embodiment 3
[0063] Embodiment 3, the preparation of sulfate:
[0064] Get the etimicin sulfate crude product produced by the prior art, add water to dissolve, load the sample on HD-2 weakly acidic cation exchange resin column, use water respectively, ammoniacal liquor gradient elution, TLC detection (developing agent: chloroform-methanol-ammonia liquor , 1:1:1, lower layer, I2) and combined fractions to obtain impurity-enriched fractions. The impurity-rich fractions were separated by column chromatography with silica gel H (chloroform:methanol:ammonia water (2:1:1, lower layer) and CM-Sephadex C-25 repeatedly, and the sixth fraction was collected and concentrated. to 200-500mg / ml, adjust the pH to 4.0-5.0 with 5mol / L sulfuric acid, then add 2%-5% activated carbon, stir and decolorize at 60-80°C for 30 minutes, filter, and freeze-dry the filtrate to obtain the substance Sulfate solids.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com