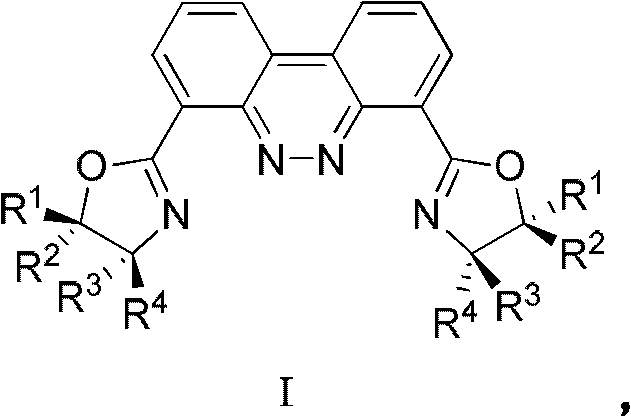
Two-oxazoline contained chiral dinuclear ligand with benzo cinnoline maternal skeleton, and synthesis method thereof
A technology of benzocinnoline and synthesis method, which is applied in the field of chiral dinuclear ligand containing two oxazolines and its synthesis, and can solve the problems of limited chiral catalytic reaction, small atomic radius, limited oxygen coordination ability, etc. , to achieve the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
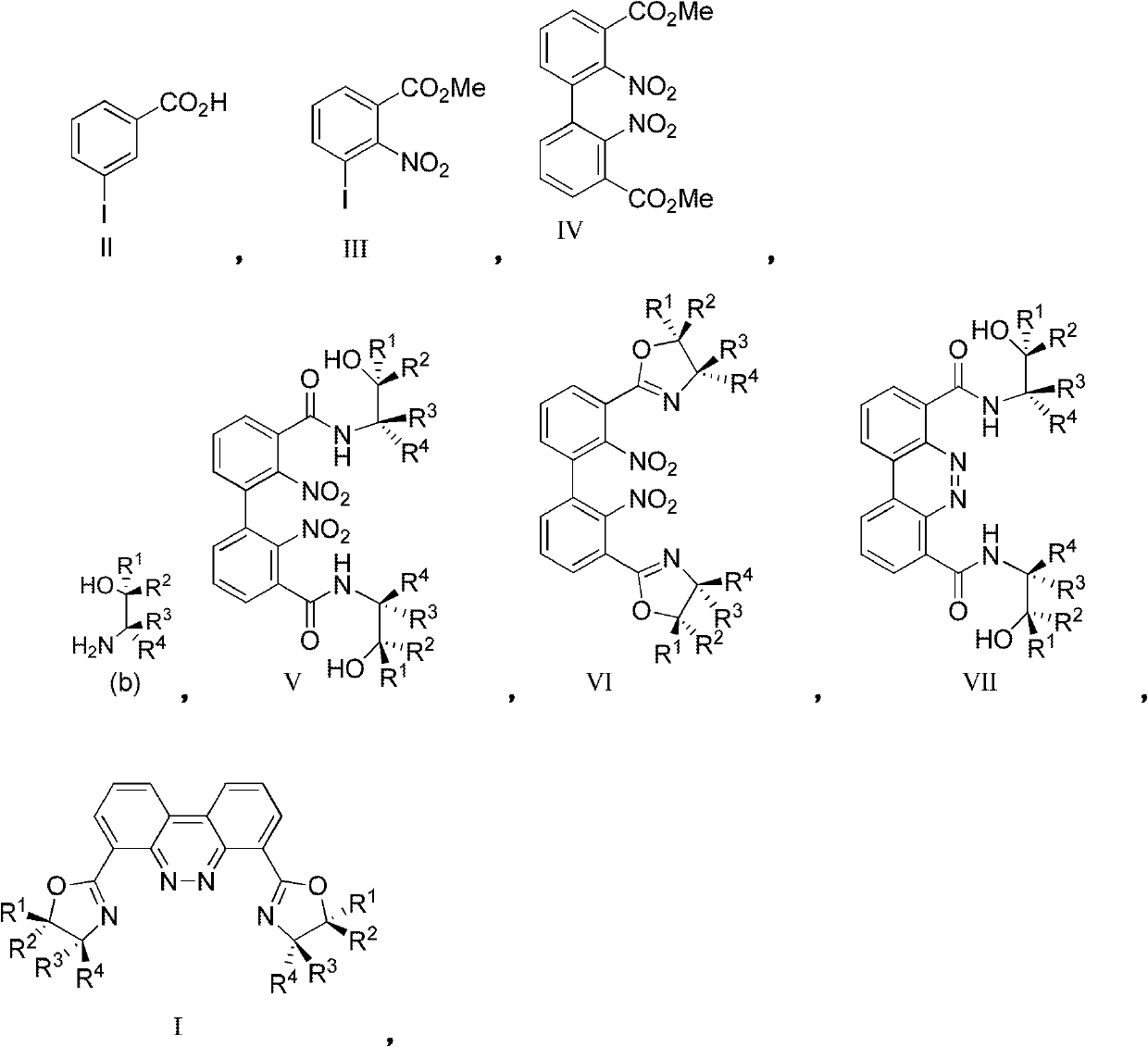
[0043] (1) prepare 2-nitro-3-iodobenzoic acid methyl ester from 3-iodobenzoic acid:
[0044] Put 6.0mL of fuming nitric acid in a 25mL two-necked bottle, drop to -10°C, slowly add 5.0g (20mmol) of m-iodobenzoic acid, react at -10°C for 3 hours, suction filter with a sand core funnel, wash with water, transfer the solid to The one-necked bottle was vacuum pumped dry. 4.28 g of a light yellow powder solid mixture was obtained.
[0045] 4.28g (14.6mmol) of the obtained light yellow powder solid mixture was dissolved in 11.6mL (146mmol) of methanol, 2mL of concentrated sulfuric acid was added dropwise, heated to 70°C, and the reaction was completed in 24 hours. Add 40 mL each of water and ethyl acetate dropwise, separate the layers, dry the organic phase with anhydrous sodium sulfate, spin dry, and separate through a column. The product 2-nitro-3-iodobenzoic acid methyl ester was obtained as 2.06 g of white crystals, and the yield was 33.5%.
[0046] 1H NMR (400MHz, CDCl 3 )δ=...
Embodiment 2
[0063] (1) Preparation of compound 2-nitro-3-iodobenzoic acid methyl ester from 3-iodobenzoic acid:
[0064] Put 10.0mL of fuming nitric acid in a 25mL two-necked bottle, drop to -10°C, slowly add 5.0g (20mmol) of m-iodobenzoic acid, react at -5°C for 100 hours, filter with a sand core funnel, wash with water, transfer the solid to The one-necked bottle was vacuum pumped dry. 4.5 g of a light yellow powder solid mixture was obtained.
[0065] 4.28g (14.6mmol) of the obtained light yellow powder solid mixture was dissolved in 0.85mL (14.6mmol) of methanol, 2mL of concentrated sulfuric acid was added dropwise, heated to 65°C, and the reaction was completed in 100 hours. Add 40 mL each of water and ethyl acetate dropwise, separate the layers, dry the organic phase with anhydrous sodium sulfate, spin dry, and separate through a column. The product 2-nitro-3-iodobenzoic acid methyl ester was obtained as 1.5 g of white crystals, and the yield was 24.4%.
[0066] 1H NMR (400MHz, C...
Embodiment 3
[0082] Starting from substituted m-iodobenzoic acid II, dinuclear ligand I is prepared via compounds III, IV, V, VI, VII:
[0083] (1) prepare 2-nitro-3-iodobenzoic acid methyl ester from 3-iodobenzoic acid:
[0084] Put 16.0mL of fuming nitric acid in a 25mL two-necked bottle, drop to -10°C, slowly add 5.0g (20mmol) of m-iodobenzoic acid, react at 10°C for 1 hour, filter with a sand core funnel, wash with water, and transfer the solid into a single-port Vacuum pump the bottle dry. 4.1 g of a light yellow powder solid mixture was obtained.
[0085] 4.28 g (14.6 mmol) of the obtained light yellow powder solid mixture was dissolved in 85 mL (1460 mmol) of methanol, 2 mL of concentrated sulfuric acid was added dropwise, and the reaction was carried out at 20° C. for 1 hour. Add 40 mL each of water and ethyl acetate dropwise, separate the layers, dry the organic phase with anhydrous sodium sulfate, spin dry, and separate through a column. The product 2-nitro-3-iodobenzoic acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com